Avicenna J Environ Health Eng. 10(2):72-77.
doi: 10.34172/ajehe.5402
Original Article
Cefixime Removal from Aqueous Solutions by Granular Ferric Oxide (GFO): Modeling and Optimization
Mahdi Asadi-Ghalhari 1  , Fatemeh Ranjdoost 2, *
, Fatemeh Ranjdoost 2, *  , Fatemeh Sadat Tabatabaei 3
, Fatemeh Sadat Tabatabaei 3  , Roqiyeh Mostafaloo 4
, Roqiyeh Mostafaloo 4  , Hassan Izanloo 5
, Hassan Izanloo 5  , Nasim Ghafouri 6
, Nasim Ghafouri 6  , Alireza Omidi Oskouei 7, Somaye Behnamipour 5
, Alireza Omidi Oskouei 7, Somaye Behnamipour 5  , Reza Ansari 8
, Reza Ansari 8 
Author information:
1Department of Environmental Health Engineering, Faculty of Health, Research Center for Environmental Pollutants, Qom University of Medical Sciences, Qom, Iran
2Student Research Committee, Qom University of Medical Sciences, Qom, Iran
3Department of Environmental Health Engineering, Faculty of Health, Qom University of Medical Sciences, Qom, Iran
4Department of Environmental Health Engineering, School of Public Health and Research Center for Health Sciences, Student Research Committee, Hamadan University of Medical Sciences, Hamadan, Iran
5Research Center for Environmental Pollutants, Qom University of Medical Sciences, Qom, Iran
6Department of Environmental Health Engineering, Alborz University of Medical Sciences, Alborz, Iran
7Department of Public Health, Faculty of Health, Qom University of Medical Sciences, Qom, Iran
8Monitoring Center for Water and Sewage Quality, Department of Water and Wastewater, Qom, Iran
Abstract
In recent years, the presence of various pharmaceutical residues such as cefixime (CFX) in aquatic environments has been gaining attention due to its adverse effects on health and ecosystems. Since conventional treatment methods are unable to remove antibiotics, sustainable and efficient approaches are needed to remove these compounds from aquatic environments. In this study, granular ferric oxide (GFO) was used to remove CFX, and the experiments were designed using Design Expert software. The findings were then analyzed using ANOVA test. The results showed that the proposed regression model fit the experimental condition (R2=0.9701, R2adjusted=0.9432, R2predicted=0.83). Several residual plots were used to confirm the suitability of the model. The initial concentration of 1.84 mg/L, GFO dose of 3.05 mg/L, and contact time of 24.32 minutes were found to be the ideal conditions for CFX adsorption. Moreover, the findings showed that GFO can be effective in absorbing and removing CFX from aqueous environments.
Keywords: Granular ferric oxide, Cefixime, Adsorption, Aqueous solutions,
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Asadi-Ghalhari M, Ranjdoost F, Tabatabaei FS, Mostafaloo R, Izanloo H, Ghafouri N, et al. Cefixime removal from aqueous solutions by granular ferric oxide (GFO): modeling and optimization. Avicenna J Environ Health Eng. 2023; 10(2):72-77. doi:10.34172/ajehe.5402
1. Introduction
Recently, the presence of various pharmaceutical residues such as cefixime (CFX) in aquatic environments has been attracting attention due to its adverse effects on health and ecosystems (1). Human excrement, hospital waste, veterinary waste, and pharmaceutical waste are the sources of pharmaceutical compounds entering the environment (2,3). In addition, the efficiency of conventional wastewater treatment to reduce pharmaceutical residues is low; therefore, widespread misuse of drugs, especially antibiotics, causes these compounds to enter the environment (4).
Antibiotics belong to a group of medicines which are used to control bacterial infections (5). Their presence in the environment can increase genotoxicity, antibiotic resistance, and toxicity in the food chain (6). Antibiotic resistance can cause the death of 700 000 people in the world every year (7). CFX is a type of antibiotic from the cephalosporin class. CFX is effective against various bacterial infections. It has been reported that 40% to 50% of the ingested CFX is removed by urination (8,9). In addition, CFX is the most prevalent antibiotic detected in aquatic environments with a maximum concentration of 422.1 ng/L (10).
There are physical (filtration, adsorption, and coagulation/flocculation), chemical (ion exchange and advanced oxidation processes), and biological methods to eliminate or degrade CFX (11,12). The biological treatment cannot effectively degrade antibiotics such as CFX because this process destroys the beneficial microorganisms involved in biological wastewater treatment (13). Furthermore, membrane and advanced oxidation processes are expensive on a large scale (14). The adsorption process removes CFX from pharmaceutical wastewater more effectively than other physical methods (15). The advantages of the adsorption process include non-toxic residual production, pollutant recovery and reuse, low design cost, suitability for batch and continuous processes, and ease of operation (16). The adsorbent type, pollutant characteristics, and type of wastewater are important parameters affecting the efficiency of the adsorption process (17). In recent years, adsorbents made of organic and inorganic materials have been used to remove various pollutants from aquatic environments (16), including nanocomposite BiFeO3 (18), activated carbon, nanosilica synthesized from rice husk (19), and iron-based adsorbents such as granular ferric hydroxide (GFH) (20) and granular ferric oxide (GFO) (21). Among the advantages of these two adsorbents, mention can be made of high potential removal efficiency (up to 98%), easy operation, low cost, no residue of waste sludge, and simplicity of composition (22).
Response surface methodology (RSM) is a technique employed in the optimization of variables and the interaction between dependent and independent variables. Generally, RSM is adopted for experimental design (23). In previous studies, it has been found that GFO can remove heavy metals from aquatic environments, but research on the removal of organic compounds with this adsorbent is scant. As such, the present study was conducted to investigate the absorption of CFX by GFO with the effect of parameters of initial CFX concentration, dose of GFO, and contact time. Towards this aim, GFO was used and the process was optimized using RSM.
2. Material and Methods
2.1. Preparation of Solutions
The compounds used in this study were all of the analytical grades. The stock solution of CFX (1 g/L) was obtained by adding 0.01 g of CFX powder (C16H15N5O7S2 (0.98%), Merck, Germany) to 10 mL of methanol. Then, the as-prepared stock solution was diluted to achieve the necessary concentrations. The fresh stock solution was prepared for each series of runs. A spectrophotometer (Cecil 7250) at a wavelength of 288.5 nm was used to measure CFX. Then, CFX removal efficiency was calculated using equation 1:
Where Ci: initial concentrations, Cf: final concentrations, RE (%): removal efficiency of CFX (%)
2.2. Preparation of GFO
The adsorbent was prepared according to the method used in the study by Tabatabaei et al (21). Electrolysis was utilized to prepare GFO. Iron electrodes were used to produce these granules. After the sediment was formed, they were rinsed several times with deionized water. Afterwards, it was dried in a furnace at a temperature of 600°C in order to produce ferric oxide.
2.3. Design and Modeling
Central Composite Design (CCD) was used to analyze, estimate, and optimize the efficacy of GFO in the removal of CFX considering three main factors, namely, initial concentration of CFX (mg/L), dose of GFO (mg/L), and contact time (minute). The CCD-based runs conducted by Design Expert software were used as the basis for the experiments. The range of contributions made by the factors and their levels are summarized in Table 1. Additionally, Table 2 displays the matrix of experiments and CCD results.
Table 1.
Symbol and Code Level of Variables
|
Variables
|
Symbol
|
Code Level
|
|
-1
|
-α
|
0
|
+α
|
+1
|
| Initial concentration of CFX (mg/L) |
A |
1 |
1.7 |
2.75 |
3.79 |
4.5 |
| Dose of GFO (g/L) |
B |
0 |
1.01 |
2.5 |
3.98 |
5 |
| Contact time (min) |
C |
5 |
13.1 |
25 |
36.89 |
45 |
Table 2.
Designing Experiments with CCD and Determining the Removal Factors and Efficiency of Each Run
|
Experimental Design
|
Removal Efficiency (%)
|
|
Run
|
Initial Concentration of CFX (mg/L)
|
Dose of GFO (g/L)
|
Contact Time (min)
|
R%
|
Predicted
|
Residual
|
| 1 |
1.7 |
3.98 |
36.89 |
69.6 |
72.19 |
2.59 |
| 2 |
2.75 |
2.5 |
25 |
63.16 |
66.49 |
3.33 |
| 3 |
2.75 |
2.5 |
45 |
70.7 |
70.63 |
-0.07 |
| 4 |
2.75 |
2.5 |
25 |
65.37 |
66.49 |
1.12 |
| 5 |
4.5 |
2.5 |
25 |
80.4 |
82.15 |
1.75 |
| 6 |
1.7 |
1.01 |
13.1 |
49.1 |
47.95 |
-1.15 |
| 7 |
1 |
2.5 |
25 |
85.2 |
83.83 |
-1.37 |
| 8 |
2.75 |
0 |
25 |
0 |
23.31 |
23.31 |
| 9 |
3.79 |
1.01 |
13.1 |
50.1 |
46.34 |
-3.76 |
| 10 |
1.7 |
1.01 |
36.89 |
78.1 |
77.39 |
-0.71 |
| 11 |
2.75 |
2.5 |
25 |
70.56 |
66.49 |
-4.07 |
| 12 |
2.75 |
2.5 |
5 |
41.6 |
42.23 |
0.63 |
| 13 |
3.79 |
3.98 |
13.1 |
69.2 |
70.07 |
0.87 |
| 14 |
2.75 |
5 |
25 |
52.2 |
47.05 |
-5.15 |
| 15 |
2.75 |
2.5 |
25 |
65.8 |
66.49 |
0.69 |
| 16 |
2.75 |
2.5 |
25 |
66.1 |
66.49 |
0.39 |
| 17 |
2.75 |
2.5 |
25 |
67.2 |
66.49 |
-0.71 |
| 18 |
3.79 |
1.01 |
36.89 |
57.3 |
54.63 |
-2.67 |
| 19 |
1.70 |
3.98 |
13.1 |
44 |
47.30 |
3.30 |
| 20 |
3.79 |
3.98 |
36.89 |
70.1 |
70.93 |
0.83 |
3. Results and Discussion
3.1. Characterization of GFO
SEM analysis was performed using a HITACHI S-4160 microscope and a 30 kV acceleration voltage was used to characterize the morphology of GFO. The magnification ranged from 20 to 3000, with a working distance of 5 nm. The GFO image is shown in Fig. 1. Tabatabaei et al (21) presented the FTIR spectra of GFO.

Fig. 1.
SEM Images of GFO
.
SEM Images of GFO
3.2. Model Fitness and Validation
Table 3 presents the results of CCD for CFX adsorption on GFO. Additionally, response graphs were generated by Design Expert. To see how the process factors and responses interacted, the analysis of variance (ANOVA) was used to visually analyze the data. Given the highest R2 value and complete lack of fit, the CCD selected the quadratic model as the best-fitted model (Table 3). For this investigation, the coefficient of variance was 10.35%. The modified R2 (0.9432) and the expected R2 are reasonably in agreement (the difference is less than 0.2). The model is implied to be significant by the model F-value of 36.08. Because of these factors, there is only a 0.01% chance that a significant F-value will occur.
Table 3.
ANOVA for the CFX Elimination Using GFO Second-order Model
|
Source
|
Sum of Squares
|
df
|
Mean Square
|
F
Value
|
P
Value
|
|
| Model |
5.66E + 07 |
9 |
6.29E + 06 |
36.08 |
< 0.0001 |
Significant |
| A-initial concentration of CFX |
93804.33 |
1 |
93804.33 |
0.5384 |
0.4799 |
|
| B-dose of GFO |
3.37E + 06 |
1 |
3.37E + 06 |
19.32 |
0.0013 |
|
| C- contact time |
1.24E + 07 |
1 |
1.24E + 07 |
71.16 |
< 0.0001 |
|
| AB |
3.99E + 06 |
1 |
3.99E + 06 |
22.89 |
0.0007 |
|
| AC |
4.07E + 06 |
1 |
4.07E + 06 |
23.37 |
0.0007 |
|
| BC |
2.56E + 05 |
1 |
2.56E + 05 |
1.47 |
0.2535 |
|
| A2 |
1.10E + 07 |
1 |
1.10E + 07 |
62.93 |
< 0.0001 |
|
| B2 |
1.67E + 07 |
1 |
1.67E + 07 |
95.7 |
< 0.0001 |
|
| C2 |
1.93E + 06 |
1 |
1.93E + 06 |
11.07 |
0.0077 |
|
| Residual |
1.74E + 06 |
10 |
1.74E + 05 |
|
|
|
| Lack of fit |
1.20E + 06 |
5 |
2.40E + 05 |
2.23 |
0.2002 |
Not significant |
| Pure error |
5.40E + 05 |
5 |
1.08E + 05 |
|
|
|
| Cor Total |
5.83E + 07 |
19 |
|
|
|
|
R2 = 0.9701, R2adjusted = 0.9432, R2predicted = 0.83, AP = 21.9684, coefficient of variation = 10.35%
Model terms are considered significant when the P value is less than 0.0500. In this model, it was discovered that AB, AC, A2, B2, and C2 had a substantial impact on how CFX absorbed on GFO. The signal-to-noise ratio was measured by Adeq Precision. A signal-to-noise ratio of at least 4 is ideal because it denotes a strong signal. To move around the design space, this model was used. The distribution of data was brought closer to normal distribution by the Box-Cox diagram. The optimal values of Lambda and constant K for this test were determined to be 2 and 0.09, respectively. A quadratic model with regression coefficients is also presented in interaction equation 2.
(2)
3.3. CFX Adsorption and Process Parameters
To determine the effect of variables on CFX removal, perturbation plot and 3D contours were used. Fig. 2 shows the perturbation plot. In the perturbation plot, the sensitivity of each factor in the absorption process is shown by the slope of the lines. Based on this graph, C (contact time) had the highest positive effect on the removal rate.
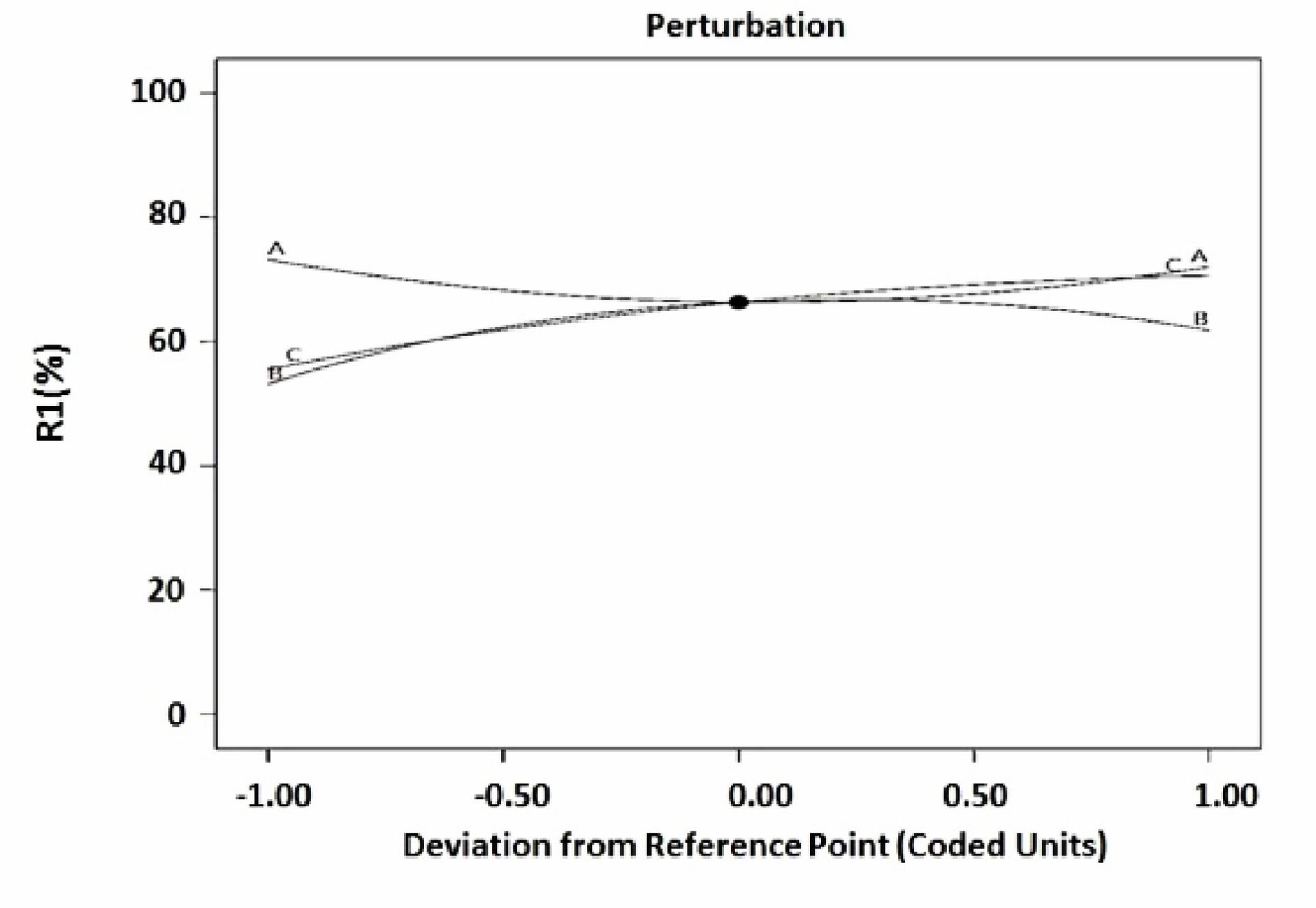
Fig. 2.
Perturbation Plots for CFX Removal Efficiency (A) Initial Concentration of CFX (B) Dose of GFO (C) Contact Time
.
Perturbation Plots for CFX Removal Efficiency (A) Initial Concentration of CFX (B) Dose of GFO (C) Contact Time
Figs. 3A and 3B show the interaction of the initial CFX concentration with contact time and initial CFX concentration with adsorbent dose, respectively. Fig. 3A illustrates the effect of interaction between the initial concentration of CFX and the contact time. According to this graph, there is a positive correlation between the amount of pollutant removal and the duration of contact time. Additionally, by increasing the concentration of CFX up to 2.75, the rate of removal of this pollutant decreased, leading to an increase in the rate of removal. The rapid removal of CFX can be attributed to the large number of active sites on the surface of the adsorbent (21). In the study by Kais and Yeddou-Mezenner, the removal of rifampicin increased with increasing contact time at different pH values of the solution (24). Likewise, in the research conducted by Yegane et al, by increasing the contact time from 0 to 90 minutes, the removal rate of ceftriaxone increased with the help of activated carbon modified with magnetite Fe3O4 nanoparticles (25).
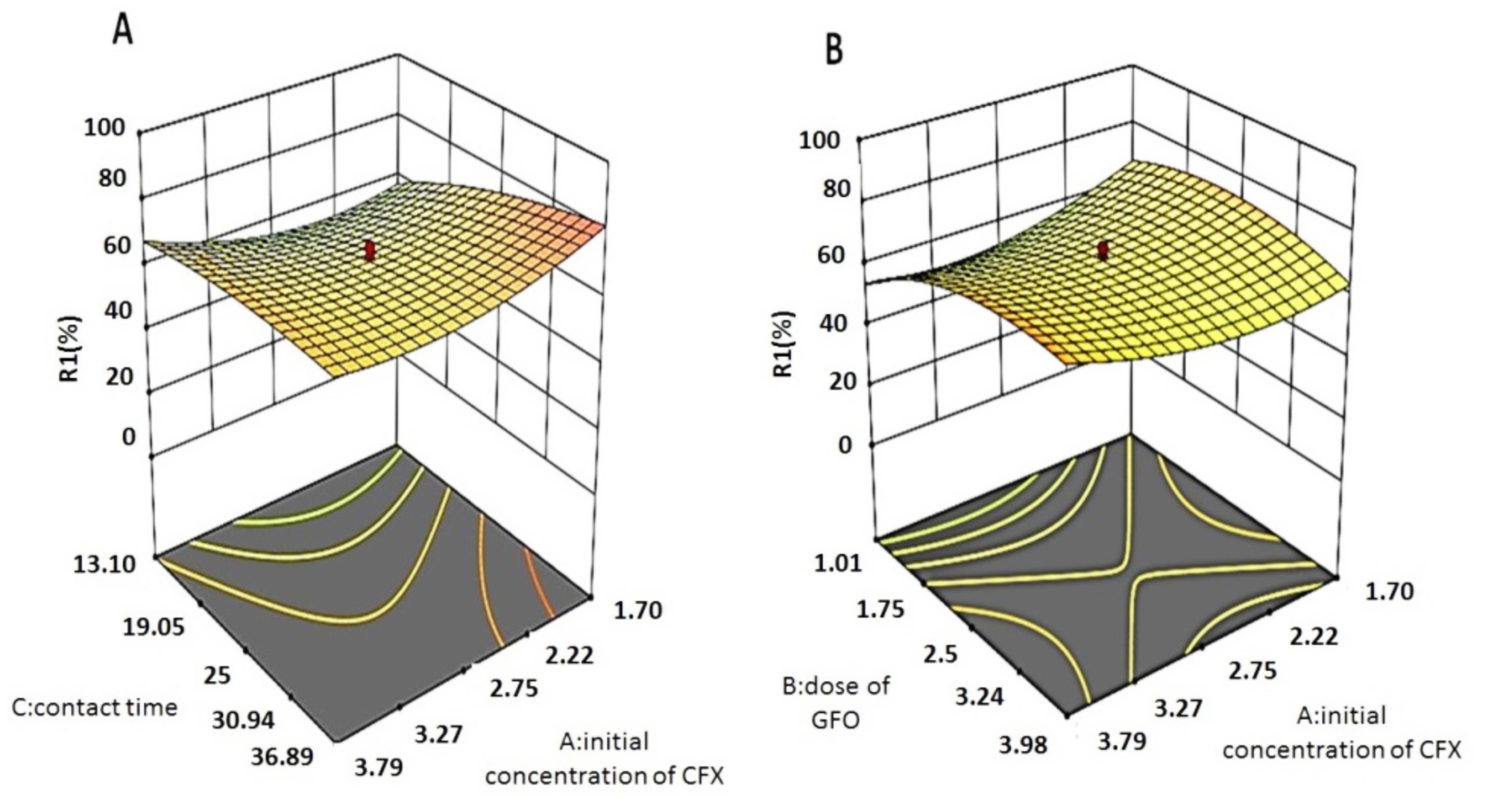
Fig. 3.
Response Surface Plots for CFX Removal as a Function of A) Cefixime-Contact time B) Cefixime-adsorbent Dose
.
Response Surface Plots for CFX Removal as a Function of A) Cefixime-Contact time B) Cefixime-adsorbent Dose
As can be observed in Fig. 3B, by increasing the adsorbent dose from 1.01 to 2.5 g/L, the amount of pollutant removal increased. The removal rate increased along with an increase in the initial CFX concentration. In their research, Mostafaloo et al used GFH to remove CFX. They reported the concentration range of CFX and the optimal concentration to be 1-15 mg/L and 8 mg/L, respectively (20). The concentration of CFX in the study by Mostafaloo et al was higher than that in the present study, which explains the reason behind the non-reduction of removal efficiency by increasing CFX concentration due to the fact that the adsorbent capacity had not reached the equilibrium level. Furthermore, the decrease in pollutant removal with increasing adsorbent dose can be attributed to the increase in the level of adsorption relative to a certain amount. Over time, the adsorption of the pollutant decreases, which is ascribed to the saturation of the active sites in the adsorbent (26). In the study by Moridi et al, increasing the adsorbent dose of Superb nZVI/Copper Slag nanocomposite increased CFX removal (27). Along the same lines, in the research by Hemmat et al, the removal rate of cephalexin increased by increasing the dose of activated carbon (28).
3.4. Optimization
One of the applications of RSM is to determine the optimum conditions. In this research, the optimal predicted removal efficiency (69.68%) was achieved at the initial concentration of 18.42 mg/L, dose of 3.05 g/L, and contact time of 24.32 minutes using the developed model (Fig. 4).
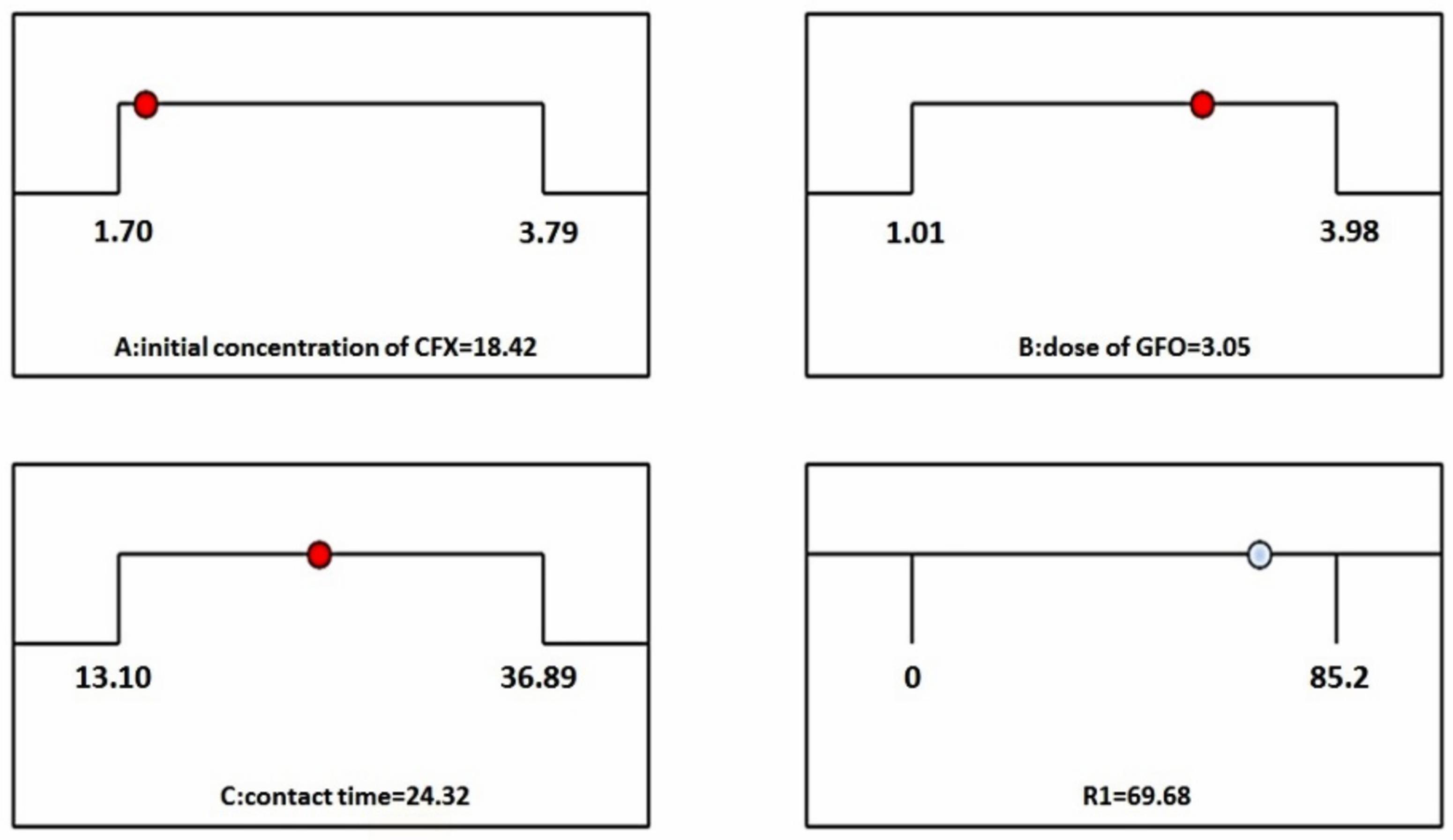
Fig. 4.
Desirability Ramp for Optimization of Central Composite with RSM
.
Desirability Ramp for Optimization of Central Composite with RSM
4. Conclusion
In this study, CFX removal was carried out using GFO. RSM was used to save time and expense. The results of the present study showed that GFO can effectively remove CFX from aqueous solution. It is noteworthy that the obtained model for data interpretation was quadratic. The optimal factors obtained for CFX decomposition were as follows: initial concentration of 1.84 mg/L, GFO dose of 3.05 mg/L, and time of 24.32 minutes. In addition, the model used in the study predicted the removal efficiency at the optimal point with a desirability of 68.69. Considering the proper removal of CFX with iron oxide granules, it is recommended that GFO should be used to remove CFX.
Acknowledgments
The authors wish to express their sincere gratitude to Qom University of Medical Sciences for supporting the current study.
Authors’ Contribution
Conceptualization: Mahdi-Asadi Ghalhari.
Data curation: Fatemeh Ranjdoost.
Formal analysis: Fatemeh Sadat Tabatabaeii.
Funding acquisition: Fatemeh Ranjdoost.
Investigation: Fatemeh Sadat Tabatabaeii, Roqieh Mostafaloo.
Methodology: Hassan Izanloo.
Project administration: Fatemeh Ranjdoost.
Resources: Somaye Behnamipour.
Software: Fatemeh Sadat Tabatabaeii, Roqieh Mostafaloo.
Supervision: Mahdi Asadi-Ghalhari, Reza AnsarI.
Validation: Mahdi Asadi-Ghalhari.
Laboratory: Nasim Ghafouri, Somaye Behnamipour.
Visualization: Roqiyeh Mostafaloo.
Writing–original draft: Fatemeh Ranjdoost.
Writing–review & editing: Alireza Omidi Oskouei.
Competing Interests
None.
Funding
The authors received no financial support for the research and/or authorship of this article.
References
- Almasi A, Dargahi A, Mohammadi M, Azizi A, Karami A, Baniamerian F. Application of response surface methodology on cefixime removal from aqueous solution by ultrasonic/photooxidation. Int J Pharm Technol 2016; 8(3):16728-36. [ Google Scholar]
- Samadi-Maybodi A, Rahmati A. Synthesis and characterization of dual metal zeolitic imidazolate frameworks and their application for removal of cefixime. J Coord Chem 2019; 72(18):3171-82. doi: 10.1080/00958972.2019.1682562 [Crossref] [ Google Scholar]
- Ciğeroğlu Z, Küçükyıldız G, Erim B, Alp E. Easy preparation of magnetic nanoparticles-rGO-chitosan composite beads: optimization study on cefixime removal based on RSM and ANN by using genetic algorithm approach. J Mol Struct 2021; 1224:129182. doi: 10.1016/j.molstruc.2020.129182 [Crossref] [ Google Scholar]
- Almasi A, Mohammadi M, Baniamerian F, Berizi Z, Almasi MH, Pariz Z. Modeling of antibiotic degradation in sonophotocatalytic process, increasing biodegradability and process optimization by response surface methodology (RSM). Int J Environ Sci Technol 2019; 16(12):8437-48. doi: 10.1007/s13762-019-02307-5 [Crossref] [ Google Scholar]
- Carabineiro SA, Thavorn-amornsri T, Pereira MF, Serp P, Figueiredo JL. Comparison between activated carbon, carbon xerogel and carbon nanotubes for the adsorption of the antibiotic ciprofloxacin. Catal Today 2012; 186(1):29-34. doi: 10.1016/j.cattod.2011.08.020 [Crossref] [ Google Scholar]
- Ahmad A, Dutta J. The role of combination beads for effective removal of antibiotic cefixime from water: towards of better solution. J Phys Conf Ser 2020; 1531(1):012092. doi: 10.1088/1742-6596/1531/1/012092 [Crossref] [ Google Scholar]
- Liu M, Ni H, Yang L, Chen G, Yan X, Leng X. Pretreatment of swine manure containing β-lactam antibiotics with whole-cell biocatalyst to improve biogas production. J Clean Prod 2019; 240:118070. doi: 10.1016/j.jclepro.2019.118070 [Crossref] [ Google Scholar]
- Wang L, Wang C, Liu Q, Meng Q, Huo X, Sun P. PEPT1- and OAT1/3-mediated drug–drug interactions between bestatin and cefixime in vivo and in vitro in rats, and in vitro in human. Eur J Pharm Sci 2014; 63:77-86. doi: 10.1016/j.ejps.2014.06.019 [Crossref] [ Google Scholar]
- Zhang T, Zhou R, Wang P, Mai-Prochnow A, McConchie R, Li W. Degradation of cefixime antibiotic in water by atmospheric plasma bubbles: performance, degradation pathways and toxicity evaluation. Chem Eng J 2021; 421(Pt 2):127730. doi: 10.1016/j.cej.2020.127730 [Crossref] [ Google Scholar]
- Mirzaei R, Yunesian M, Nasseri S, Gholami M, Jalilzadeh E, Shoeibi S. Occurrence and fate of most prescribed antibiotics in different water environments of Tehran, Iran. Sci Total Environ 2018; 619-620:446-59. doi: 10.1016/j.scitotenv.2017.07.272 [Crossref] [ Google Scholar]
- Wang W, Fang J, Chen H, Bao N, Lu C. Rice-husk-derived mesoporous 0D/2D C3N4 isotype heterojunction with improved quantum effect for photodegradation of tetracycline antibiotics. Ceram Int 2019; 45(2 Pt A):2234-40. doi: 10.1016/j.ceramint.2018.10.136 [Crossref] [ Google Scholar]
- Shaniba C, Akbar M, Ramseena K, Raveendran P, Narayanan BN, Ramakrishnan RM. Sunlight-assisted oxidative degradation of cefixime antibiotic from aqueous medium using TiO2/nitrogen doped holey graphene nanocomposite as a high performance photocatalyst. J Environ Chem Eng 2020; 8(1):102204. doi: 10.1016/j.jece.2018.02.012 [Crossref] [ Google Scholar]
- Cigu TA, Vasiliu S, Racovita S, Lionte C, Sunel V, Popa M. Adsorption and release studies of new cephalosporin from chitosan-g-poly(glycidyl methacrylate) microparticles. Eur Polym J 2016; 82:132-52. doi: 10.1016/j.eurpolymj.2016.07.011 [Crossref] [ Google Scholar]
- Pouretedal HR, Sadegh N. Effective removal of amoxicillin, cephalexin, tetracycline and penicillin G from aqueous solutions using activated carbon nanoparticles prepared from vine wood. J Water Process Eng 2014; 1:64-73. doi: 10.1016/j.jwpe.2014.03.006 [Crossref] [ Google Scholar]
- Nazari G, Abolghasemi H, Esmaieli M. Batch adsorption of cephalexin antibiotic from aqueous solution by walnut shell-based activated carbon. J Taiwan Inst Chem Eng 2016; 58:357-65. doi: 10.1016/j.jtice.2015.06.006 [Crossref] [ Google Scholar]
- Ibisi NE, Asoluka CA. Use of agro-waste (Musa paradisiaca peels) as a sustainable biosorbent for toxic metal ions removal from contaminated water. Chem Int 2018; 4(1):52-9. [ Google Scholar]
- Naghipour D, Amouei A, Estaji M, Taghavi K, Allahabadi A. Cephalexin adsorption from aqueous solutions by biochar prepared from plantain wood: equilibrium and kinetics studies. Desalin Water Treat 2019; 143:374-81. [ Google Scholar]
- Mostafaloo R, Mahmoudian MH, Asadi-Ghalhari M. BiFeO3/Magnetic nanocomposites for the photocatalytic degradation of cefixime from aqueous solutions under visible light. J Photochem Photobiol A Chem 2019; 382:111926. doi: 10.1016/j.jphotochem.2019.111926 [Crossref] [ Google Scholar]
- Chen Y, Wang F, Duan L, Yang H, Gao J. Tetracycline adsorption onto rice husk ash, an agricultural waste: its kinetic and thermodynamic studies. J Mol Liq 2016; 222:487-94. doi: 10.1016/j.molliq.2016.07.090 [Crossref] [ Google Scholar]
- Mostafaloo R, Asadi-Ghalhari M, Aali R, Tabatabaei FS, Sadat E, Kishipour A. Application of response surface methodology for optimization of cefixime removal from aqueous solutions by granular ferric hydroxide. J Environ Treat Tech 2020; 8(3):1112-7. [ Google Scholar]
- Tabatabaei FS, Izanloo H, Heidari H, Vaezi N, Zamanzadeh M, Nadali A. Modeling and optimization of arsenic(III) removal from aqueous solutions by GFO using response surface methodology. Pollution 2020; 6(3):543-53. doi: 10.22059/poll.2020.296452.739 [Crossref] [ Google Scholar]
- Singh R, Singh S, Parihar P, Singh VP, Prasad SM. Arsenic contamination, consequences and remediation techniques: a review. Ecotoxicol Environ Saf 2015; 112:247-70. doi: 10.1016/j.ecoenv.2014.10.009 [Crossref] [ Google Scholar]
- Yousefi M, Gholami M, Oskoei V, Mohammadi AA, Baziar M, Esrafili A. Comparison of LSSVM and RSM in simulating the removal of ciprofloxacin from aqueous solutions using magnetization of functionalized multi-walled carbon nanotubes: process optimization using GA and RSM techniques. J Environ Chem Eng 2021; 9(4):105677. doi: 10.1016/j.jece.2021.105677 [Crossref] [ Google Scholar]
- Kais H, Yeddou-Mezenner N. Studies on adsorptive removal of an antibiotic drug using ion exchange resin. Alger J Environ Sci Technol 2019; 5(2):923-9. [ Google Scholar]
- Yegane Badi M, Azari A, Pasalari H, Esrafili A, Farzadkia M. Modification of activated carbon with magnetic Fe3O4 nanoparticle composite for removal of ceftriaxone from aquatic solutions. J Mol Liq 2018; 261:146-54. doi: 10.1016/j.molliq.2018.04.019 [Crossref] [ Google Scholar]
- Ouaissa YA, Chabani M, Amrane A, Bensmaili A. Removal of tetracycline by electrocoagulation: kinetic and isotherm modeling through adsorption. J Environ Chem Eng 2014; 2(1):177-84. doi: 10.1016/j.jece.2013.12.009 [Crossref] [ Google Scholar]
- Moridi A, Sabbaghi S, Rasouli J, Rasouli K, Hashemi SA, Chiang WH. Removal of cefixime from wastewater using a superb nZVI/copper slag nanocomposite: optimization and characterization. Water 2023; 15(10):1819. doi: 10.3390/w15101819 [Crossref] [ Google Scholar]
- Hemmati M, Ghaemi A, Tavakkoli H. Removal of cephalexin antibiotic from aqueous solutions by activated carbon adsorbent. J Res Environ Health 2019;5(1):11-20. [Persian].