Avicenna J Environ Health Eng. 9(1):45-53.
doi: 10.34172/ajehe.2022.06
Original Article
Enhanced Biodegradation of Formaldehyde Using Aerobic Sequencing Batch Rotating Bed Bioreactor With and Without Stimulation by Hydrogen Peroxide
Ahmadreza Yazdanbakhsh 1, 2, *  , Mohsen Sadani 2
, Mohsen Sadani 2  , Mohammad Golaki 2
, Mohammad Golaki 2 
Author information:
1Workplace Health Promotion Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2Department of Environmental Health Engineering, School of Public Health and Safety, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Abstract
The removal of formaldehyde as a toxic substance from aqueous solutions is of particular importance. In this research, a sequencing batch rotating-bed bioreactor (SBRB) was used on a laboratory scale for biodegradation of formaldehyde from synthetic wastewater. The reactor was made of plexiglas with a cylindrical shape. Kaldnes media were placed in a rotating cylindrical basket in the reactor. The effects of formaldehyde concentration (500–1500 mg/L), hydraulic retention time (HRT) (8, 15, 24 hours), and injection of hydrogen peroxide (0.1-0.5 mM) on the performance of the reactor were investigated. The results showed that in the SBRB, at an HRT of 24 hours and an inlet formaldehyde concentration of 1000 mg/L, the removal efficiencies of formaldehyde and chemical oxygen demand (COD) were 99.2% and 92%, respectively, while without rotating the bed, the removal efficiency of formaldehyde and COD was found to be 95% and 83%, respectively. By adding hydrogen peroxide at a concentration of 0.3 mM and operation of the SBRB with an HRT of 8 hours and an inlet formaldehyde concentration of 1000 mg/L, an improvement in the removal efficiency of formaldehyde and COD (4% and 22%, respectively) was observed. Accordingly, SBRB stimulation with hydrogen peroxide could be considered as a high-performance process for the removal of formaldehyde and corresponding COD at a short HRT.
Keywords: Formaldehyde, Rotating bed, Bioreactor, Wastewater, Peroxidase
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Yazdanbakhsh A, Sadani M, Golaki M. Enhanced biodegradation of formaldehyde using aerobic sequencing batch rotating bed bioreactor with and without stimulation by hydrogen peroxide. Avicenna J Environ Health Eng. 2022; 9(1):45- 53. doi:10.34172/ajehe.2022.06
1. Introduction
Formaldehyde (CH2O) with a molecular weight of 30.03 g mol-1 is a colorless and odorless gas. This compound is widely used by some industries such as the production of synthetic resins, adhesives, leather, petrochemical products, fabrics, polyester fibers, medical and pharmaceutical products, plastics, fiberglass, wood, paper, and aquaculture products (1,2). Formaldehyde concentration in industrial wastewater can range from 0.2-4 g/L for adhesivemanufacturing industries to more than 10 g/L for the other industries. Formaldehyde is considered a dangerous and highly toxic substance that can harm humans and aquatic animals in aquatic environments (3). Industrial wastewater containing high concentrations of formaldehyde can have inhibitory effects on bacterial activity in biological processes (4). Formaldehyde can react directly with DNA, RNA, and proteins and kill microorganisms. Carcinogenicity and mutagenic effects are attributed to high concentrations of formaldehyde (5). US environmental protection agency identifies it as a potential human carcinogen (6). Therefore, discharging untreated formaldehyde-containing wastewater into aquatic environments can seriously endanger aquatic life (6,7). Therefore, in order to protect human health and ecosystems against the destructive effects of formaldehyde, it is necessary to remove it from the wastewater or reduce it to the permissible amounts of discharge (imposed by the relevant organizations) (8,9). So far, various chemical, physical, and biological methods have been studied to remove formaldehyde from wastewater (10-13).
Chemical methods such as advanced oxidation, condensation, and precipitation are usually expensive, and physical methods, such as adsorption, have some disadvantages, including the recovery and reuse of adsorbents. In the meantime, biological processes are preferred to physical and chemical methods due to their ability to remove large amounts of pollutants, environmentally friendliness, cost-effectiveness, ease of operation, and low pollution. Bioreactors play a crucial role in the biodegradation of wastewater. Studies have shown that formaldehyde is biodegradable under both aerobic and anaerobic conditions (7,9,13,14). Anaerobic microorganisms are more sensitive to formaldehyde than aerobic microorganisms, due to the formation of intermediates such as formic acid and methanol and the presence of significant amounts of COD in the effluent of anaerobic processes. Moreover, due to the limited mass transfer of formaldehyde in anaerobic biological reactors and low treatment efficiency, it is necessary to evaluate the efficiency of aerobic bioreactors in formaldehyde removal (14).
Sequencing batch reactor (SBR) is one of the most common activated sludge processes for the treatment of organic pollutants in industrial wastewater. In this process, suspended biomass is used to remove the pollutants. This system can have problems at high organic and hydraulic loads; therefore, the performance of these systems needs to be improved (15).
The SBR was modified by inserting a moving media into the bioreactor and developing a moving-bed SBR (MBSBR) which further improved performance (16). However, one of the major problems of this reactor was that the media floated on the surface of the reactor during periods of settling and decanting. This problem resulted in poor sedimentation of the effluent. In order to overcome this problem, the media was enclosed in a basket and the media-containing basket was rotated inside the reactor. The new reactor was named as the rotating bed reactor. According to studies, this reactor has shown proper capabilities for the biological treatment of industrial wastewater with toxic compounds (14,17).
Enzymatic biodegradability enhancement has been used by various researchers to degrade resistant compounds. Hydrogen peroxide is one of the compounds that has received more attention in recent years for the biostimulation of microorganisms to produce peroxidase enzyme in biological systems. Hydrogen peroxide injection leads to the production of enzyme peroxidases and free radicals and increases the biodegradability of recalcitrant compounds (18-21). The advantages of adding hydrogen peroxide are low cost, simple injection, rapid decomposition, as well as the release of oxygen into the wastewater (providing a part of the oxygen needed by the system through natural dissolution in the wastewater). It is also important to note that due to the high solubility of hydrogen peroxide in water, there is no limit to the supply of high levels of oxygen required by the bioreactor at high organic loads (22-25). Additionally, the addition of hydrogen peroxide to the activated sludge system can produce molecular oxygen and reduce aeration costs (17,18).
Considering that no study has been done regarding the use of the aerobic rotating-bed bioreactor with the addition of hydrogen peroxide for formaldehyde removal, the aim of this study was to determine the efficiency of SBRB reactor in biodegradation of high-strength formaldehyde in the presence of hydrogen peroxide. In this study, the effects of inlet formaldehyde concentrations, various HRTs, rotating and non-rotating bed systems, as well as the use of hydrogen peroxide were investigated on the removal of formaldehyde and corresponding COD.
2. Materials and Methods
2.1. Chemicals and Synthetic Formaldehyde Sample
All chemical reagents were of analytical grade. Chemicals were purchased from Sigma–Aldrich (USA), Merck (Germany), and ChemLab (Belgium). The synthetic wastewater containing formaldehyde was prepared by dissolving formalin solution containing 38% formaldehyde in tap water. Based on the concentration of corresponding COD of formaldehyde which was equal to 1000 mg/L, the detailed composition of synthetic wastewater was as follows (mg/L): COD (1000) NH4Cl (200), NaHCO3 (200), KH2PO4 (25), K2HPO4 (58), CaCl2.2H2O (50), and MgSO4.7H2O (75). The trace elements solution was also prepared by dissolving (g): FeCl3.6H2O (1.5), H3BO3 (0.15), CuSO4.5H2O (0.03), KI, 0.03, MnCl2.4H2O (0.12), NaMoO4.2H2O (0.06), and CoCl2.6H2O (0.15) in 1 L of distilled water. Then, 1 mL of this solution was added per liter of feed. The pH of the samples was maintained at 7.5 ± 0.3.
2.2. Experimental Setup
A bench scale sequencing batch rotating-bed bioreactor (SBRB) was constructed and used in this study. The reactor was made of plexiglas with a cylindrical shape. The reactor was built with an internal diameter of 14 cm, a total height of 30 cm, and a volume of 4.6 L. The schematic of the bioreactor is illustrated in Fig. 1. Reactor operating cycle consisted of feeding, reacting (aeration), settling, and discharging stages. The reaction time or HRT of the reactor was determined to be 24, 15, and 8 hours, the time of feeding and discharging of the reactor was 5 minutes, and the settling time was 60 minutes. The system was aerated with an aeration pump and using plate diffusers at the bottom of the reactor. A valve was installed at a height of 25 cm for the sampling of effluent. A drain valve was installed at a height of 8 cm from the bottom of the reactor. To drain the sludge from the reactor, an outlet valve was installed at a height of 2 cm from the bottom. A volume of Kaldnes media (with the specifications presented in (Table 1) were packed in a cylindrical perforated basket and placed inside the reactor. The media volume was 25% of the reactor volume. During the operation, the basket was rotated by an electric motor at a speed of 30 rpm. To investigate the effect of hydrogen peroxide on reactor performance, a syringe pump was used to add hydrogen peroxide to the reactor.
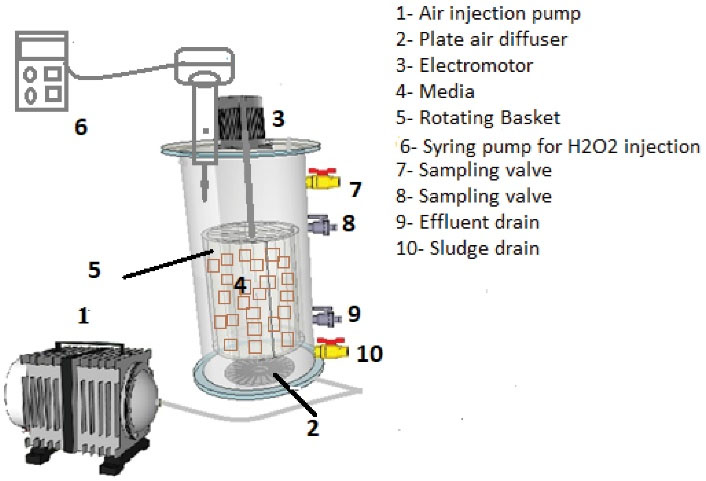
Figure 1.
Schematic of Sequencing Batch Rotating Bed Bioreactor Used in this Research.
.
Schematic of Sequencing Batch Rotating Bed Bioreactor Used in this Research.
Table 1.
Properties of Kaldnes Media
|
Parameter
|
Value
|
| Nominal special area |
600±30 m2/m3 |
| Special effective area |
m2/m3 460 |
| Volumetric density |
0.92-0.97 g/cm3 |
| Approximate diameter of each media |
30 mm |
| Material |
HDPE & PE |
2.3. Startup of System and Operation
For seeding the reactor, activated sludge was prepared from an aerobic wastewater treatment plant of the MDF board production plant. Due to the presence of formaldehyde in this wastewater (230±70 mg/L), this sludge was acclimated to formaldehyde. However, to ensure the adaptation of microorganisms to wastewater containing formaldehyde, an individual acclimation step was performed in the laboratory. The prepared sludge was aerated several times and the necessary organic matter and nutrients were added to it. The required amount of enriched sludge was added to the reactor. Initially, glucose with formaldehyde was added in equal proportions as a carbon source to the influent wastewater with a total COD of 800 mg/L. The amount of glucose was then gradually reduced to achieve a stable reactor condition in the removal of the COD by supplying the carbon formaldehyde source alone. After about 51 days, the removal efficiency of formaldehyde and COD reached the maximum and the removal trend was almost constant, so the adaptation conditions were accepted to continue the experiments. In this condition, the SBRB reactor contained mixed microbial biomass with both attached growth and suspended growth. Considering the acclimation phase, this study was conducted in 5 phases. The procedures and operational mechanisms of these phases are presented in detail in Table 2. At each stage, the condition for testing and obtaining data was to create stable conditions in the operation of the reactor. The stability condition was achieved when the rate of COD fluctuation in three consecutive cycles was negligible.
Table 2.
Experimental Phases and Operating Conditions in Each Phase
|
Phases
|
HRT
(h)
|
CH
2
O
*
Loadings (kg/m
3
.d)
|
Inlet
CH
2
O (mg/L)
|
Using Rotating Bed
|
Amount of H
2
O
2
mM
|
Operation
Days
|
| Acclimation with CH2O |
24 |
≤ 0.4 |
400 |
Yes |
0 |
1-50 |
| Effect of CH2O concentration |
24 |
0.5 -1.5 |
500 - 1500 |
Yes |
0 |
51-93 |
Effect of
HRT |
24,15,8 |
1-3 |
1000 |
Yes |
0 |
94-135 |
Effect of
rotating bed |
24 |
3 |
1000 |
NO |
0 |
136-170 |
Effect of
H2O2 injection |
8 |
3 |
1000 |
Yes |
0-0.5 |
171-220 |
2.4. Analytical Procedures
During the operation of the reactor in each phase, wastewater samples were routinely taken from the reactor and analyzed. Formaldehyde and COD concentrations were measured in effluent samples every 2 to 4 days. Depending on the system conditions, the attached and suspended biomass (MLSS) concentrations were measured every 5 to 10 days. Dehydrogenase activity was measured during the phase that hydrogen peroxide was added to the reactor (stage 4). System monitoring tests such as dissolved oxygen and pH measurements were performed daily using a YSI-155 DO meter (YSI Co., USA) and a WTW-720 pH meter (WTW CO., Germany), respectively. The colorimetric technique (Hantzsch method) was used to measure formaldehyde concentration (26). For this purpose, the DR 5000 spectrophotometer (HACH CO., USA) equipped with a visible and ultraviolet light detector was used. The absorbance of the samples was read at a maximum absorption wavelength of 412 nm. COD concentration was measured based on open reflux method according to the procedure given in standard methods (27).To determine the activity of dehydrogenase enzymes, triphenyl tetrazolium chloride salt was used in an spectrophotometric method at a wavelength of 492 nm. In this method, the amount of enzyme activity was obtained in µg, which if divided by the concentration of biomass in mg/L, the result is reported in terms of µg tri-phenylformazan (TF)/gbiomass.d. In addition, 4-amino-anti-pyrene method with a spectrophotometer at a wavelength of 510 nm was used to measure the amount of peroxidase enzyme and the result was reported in U/g biomass (17). To determine the amount of suspended biomass, 10 mL of the suspended liquid was taken from the reactor and MLSS test was performed on it. Moreover, 4 aliquots of media were withdrawn from the reactor randomly and washed with distilled water to detach biomass. The amount of biomass was then measured. According to the number of media, the total amount of attached biomass in the reactor was calculated. Total suspended and attached biomass was considered as the total MLSS for biodegradation of formaldehyde. MLSS were measured according to the procedure given in standard methods (27).
3. Results and Discussion
3.1. Bioreactor Performance in the Acclimation Phase
One of the most important steps in conducting research on the removal of toxic or inhibitory organic pollutants by biological process (as activated sludge) is the acclimation of the microorganisms with the desired pollutant. During this stage, microorganisms that have the ability to degrade the desired pollutant predominate in the reactor. During the system acclimation stage with formaldehyde, SBRB bioreactor was exploited with a carbon source including glucose and formaldehyde with a constant COD concentration of 400 mg/L (at different ratios of formaldehyde to glucose: 10, 20, 40, 50, 70, and 100%). Figs. 2a and 2b show the COD and formaldehyde removal efficiencies in SBRB bioreactor during the adaptation period, respectively. As shown in Fig. 2, despite the fact that the primary sludge used for seeding was prepared from a treatment plant of MDF board production plant, which had been in contact with wastewater containing formaldehyde, in the early days of operation, the system had low efficiency in removing formaldehyde and COD. However, the acclimation of the system occurred with high concentrations of formaldehyde shortly afterwards. From the forty-seventh day onwards, after complete removal of glucose from the influent and only feeding the reactor with formaldehyde, the system was completely acclimated for the degradation of formaldehyde. In this condition, the average COD and formaldehyde removal efficiencies in SBRB bioreactor were found to be 90 % and 99.85%, respectively. Similar to this research, in other studies, formaldehyde removal efficiency has been reported to be greater than the corresponding COD removal efficiency (13,28,29).
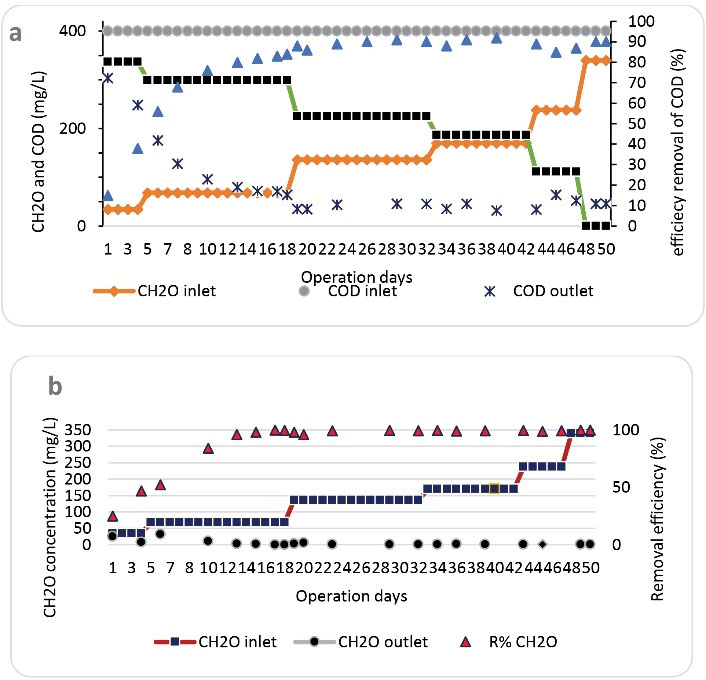
Figure 2.
Performances of the SBRB Bioreactor During the Acclimation Stage, (a) COD Removal, (b) Formaldehyde Removal (HRT=24 Hours).
.
Performances of the SBRB Bioreactor During the Acclimation Stage, (a) COD Removal, (b) Formaldehyde Removal (HRT=24 Hours).
3.2. The Influence of Formaldehyde Concentration
After acclimation of the microorganisms to formaldehyde, the effect of formaldehyde concentration on the performance of SBRB bioreactor was evaluated. In this phase, the bioreactor was operated at inlet formaldehyde concentrations of 500, 700, 1300, and 1500 mg/L. Fig. 3 shows the formaldehyde and corresponding COD removal efficiencies at different concentrations of formaldehyde at an HRT of 24 hours. In this stage, the concentration of formaldehyde entering the reactor started from 500 mg/L on the 51st day and increased to a concentration of 1500 mg/Lon the 93rd day of the reactor operation. As shown in Fig. 3, at an inlet formaldehyde concentration of 500 mg/L, the stability of the reactor performance was maintained and the removal rate of formaldehyde and COD was about 100% and 92%, respectively. Compared to the results of the previous phase, despite increasing the formaldehyde concentration from 400 to 500 mg/L, the COD removal efficiency increased. This may be due to the fact that the bacteria are more acclimated to formaldehyde, as well as the increased attached MLSS portion of biomass. With the increase of formaldehyde concentration in the following days to about 700 mg/L, formaldehyde and COD removal efficiencies with a slight decrease reached about 99 and 92%, respectively. As shown in Fig. 3, by feeding the reactor with formaldehyde at a concentration of 1000 mg/L, initially a decrease in the amount of formaldehyde and COD removal was observed; however, after a few days, the removal rate increased to about 99% and 92%, respectively. Therefore, attempts to increase the inlet formaldehyde concentration to 1300 and 1500 mg/L led to a sharper decrease in the performance of the reactor for COD removal to about 78% on the 93rd day of operation. However, the removal efficiency of formaldehyde remained at about 99%. Accordingly, formaldehyde concentrations above 1000 mg/L can have inhibitory effects on reactor performance and cause the production of high organic intermediates and the increase of concentration of COD in the effluent. So far, several studies have been performed under different conditions (the type of biological reactor, formaldehyde inlet concentration, and HRT) for the biological removal of formaldehyde. In a study by Mei et al, using a membrane-aerated biofilm reactor and an inlet formaldehyde concentration of 116 mg/L (in the presence of the co-substrate methanol), the removal rate of formaldehyde and COD reached 99.9% and 81.5%, respectively (9). Bolonhesi et al investigated the performance of combined anaerobic/aerobic SBR (ASBR-SBR) system for the removal of formaldehyde from furniture industry painting booth wastewater. The ASBR-SBR system removed 99% of COD and CH2O until the initial concentration of 180 mg/L. When the CH2O concentration increased to 232 mg/L, the removal efficiency of COD reduced to 72% in ASBR due to partial inhibition of microorganisms caused by formaldehyde toxicity. However, under these conditions, the SBR was efficient and removed the remaining organic matter by 98% (30). Moussavi and Heidarizad developed and investigated a moving-bed sequential continuous-inflow reactor (MSCR) for the degradation of high concentrations of phenol and formaldehyde. They reported that MSCR could simultaneously remove greater than 99% of the target compounds for concentrations up to 1300 mg/L and around 96% of the COD with a 6-hour cycle time (29). Wang et al used the activated sludge process for the treatment of formaldehyde wastewater. The results showed that with the initial formaldehyde concentration of 400 mg/L and HRT of 10 hours, the removal rates of formaldehyde and COD reached more than 99% and 83%, respectively. Additionally, they have reported that the maximum concentration of formaldehyde that the activated sludge process can tolerate was about 400 mg/L, and the proper reaction time was 8 hours (31). In comparison with other studies, the performance of the SBRB reactor can be considered good and significant. This suitable performance can be attributed to the active attached and suspended biomass in the reactor as well as the rotation of the media inside the reactor. The rotation of the media increased the contact of the biomass with the substrate, uniformity in the distribution of formaldehyde and oxygen in the reactor, and mass transfer.
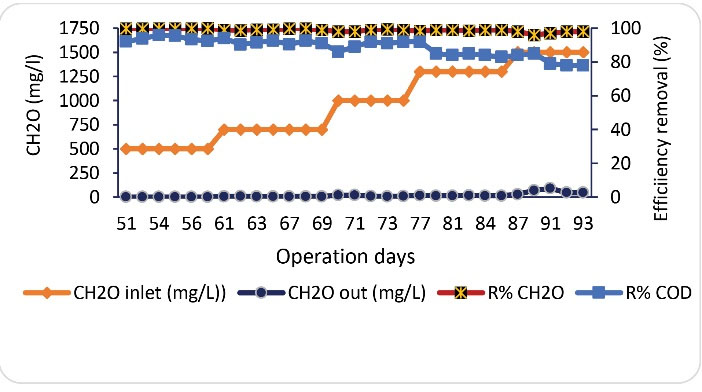
Figure 3.
Formaldehyde and Corresponding COD Removal Efficiency at Different Concentrations of Formaldehyde (HRT=24 Hours).
.
Formaldehyde and Corresponding COD Removal Efficiency at Different Concentrations of Formaldehyde (HRT=24 Hours).
3.3. The Effect of the Different Hydraulic Retention Time
In biological systems, it is important to provide adequate HRT to create opportunities for microorganisms to degrade the substrate. On the other hand, long residence time increases the volume of the reactor and thus increases the costs. Therefore, the use of a reactor that can meet the treatment needs in a shorter residence time is preferred. Therefore, in this research, the effects of HRTs of 24, 15, and 8 hours at the inlet formaldehyde concentration of 1000 mg/L on the efficiency of SBRB bioreactor were investigated. Fig. 4 shows the performances of SBRB bioreactor in the removal of COD and formaldehyde at different HRTs. As shown in Fig. 4, although by reducing the HRT from 24 hours to 15 and 8 hours, there was a slight reduction in formaldehyde removal efficiency, while the reduction in COD removal efficiency was noticeable. The efficiency of COD removal decreased from 96% (during HRT of 24 hours) to 80.6% (during HRT of 15 hours) and 56% (during HRT of 8 hours). Therefore, according to the results, at high inlet concentrations of formaldehyde in SBRB bioreactor, a minimum residence time of at least 24 hours is required to obtain high efficiencies for the simultaneous removal of formaldehyde and its corresponding COD. As it is clear, the decrease in the HRT can reduce the system efficiency. The reason for the decrease in efficiency is that there is not enough time to decompose the contaminant due to the limited capacity of decomposition of contaminants by microorganisms (9). Studies have shown that traditional reactors such as activated sludge or simple anaerobic systems require long residence times to remove formaldehyde and operation of these reactors with high concentrations of formaldehyde can cause the reactor to fail (32,33). Bolognese et al mentioned that high HRT (4 days in ASBR and 2 days in SBR) was required to remove formaldehyde. In addition, in the study by Bolognese et al, the concentration of formaldehyde was equal to 180 mg/L, which was very low compared to the present study (30). In the study by Mei et al, using a membrane-aerated biofilm reactor with a short HRT (4 hours) and an inlet formaldehyde concentration of 116 mg/L (in the presence of the co-substrate methanol), the removal rates of formaldehyde and COD reached 99.9% and 81.5%, respectively (9). However, by using high-efficiency reactors, the HRT can be greatly reduced.
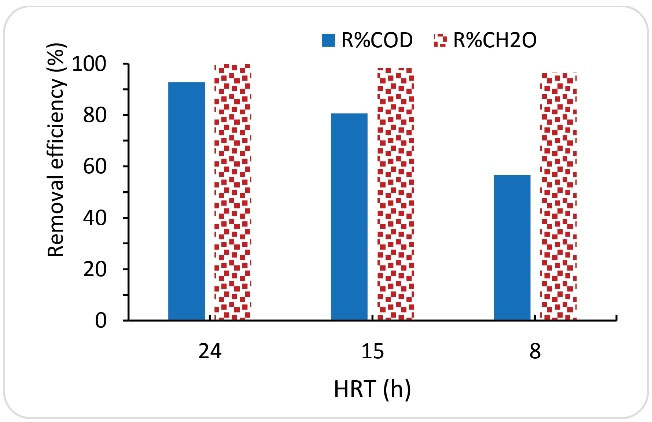
Figure 4.
Effect of HRT on the Removal of Formaldehyde and COD (Influent Formaldehyde Concentration = 1000 mg/L).
.
Effect of HRT on the Removal of Formaldehyde and COD (Influent Formaldehyde Concentration = 1000 mg/L).
3.4. The Effect of Rotating Bed on the Performance of the Reactor
In order to determine the effect of rotating bed on the reactor efficiency, at this phase, the reactor operating conditions were considered to be similar to the operating conditions of the rotating reactor with formaldehyde at an inlet concentration of 1000 mg/L and HRT of 24 hours. The only difference was that the basket containing media did not rotate in the reactor. As shown in Fig. 5, on the 136th day of operation, by rotating the bed, the removal rates of formaldehyde and COD were equal to 99.2% and 92%, respectively. In the following days, by stopping the rotating bed, the efficiency of the reactor in removing formaldehyde and COD was reduced and fluctuated. Gradually, stable conditions were achieved in the system, and on the 173rd day of operation, the removal efficiencies of formaldehyde and COD were measured to be 95% and 83%, respectively. Fig. 6 also shows the average efficiency of a reactor with and without a rotating system at each phase. As can be seen in the figure, there is a significant difference in the performance of the reactor under the two operating conditions. As previously mentioned, the rotating system of the media in the reactor environments can increase the uniformity in the distribution of formaldehyde, balance in the temperature and pH conditions in the reactor, contact of microorganisms with the substrate, and mass transfer rate. The results of these overall effects lead to an increase in the performance of the SBRB bioreactor. Other factors that reduce the efficiency of the reactor without a rotating system may be due to the effect of the environments on the edges of the reactor, as well as the poor oxygen delivery to the biofilms. Some studies have confirmed the effects of rotation and movement of media in biological reactors on the efficiency of reactors to remove contaminants from wastewater. Pourakbar et al reported that by using cyclic activated sludge integrated with a rotating bed bioreactor, at an inlet phenol concentration of 600 mg/L and HRT of 24 hours, complete biodegradation of phenol occurred (17). Moussavi et al introduced the novel MSCR as a promising technique for the degradation of high concentrations of phenol and formaldehyde mixture in a single-basin reactor. They indicated that the MSCR could simultaneously remove greater than 99% of the target compounds at concentrations up to 1300 mg/L (29).
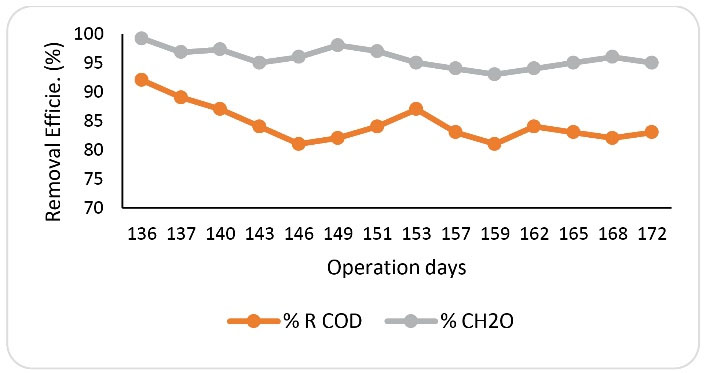
Figure 5.
Formaldehyde and COD Removal in Sequencing Batch Reactor With No Rotating (HRT=24 Hours and Inlet Concentration of CH2O = 1000 mg/L).
.
Formaldehyde and COD Removal in Sequencing Batch Reactor With No Rotating (HRT=24 Hours and Inlet Concentration of CH2O = 1000 mg/L).
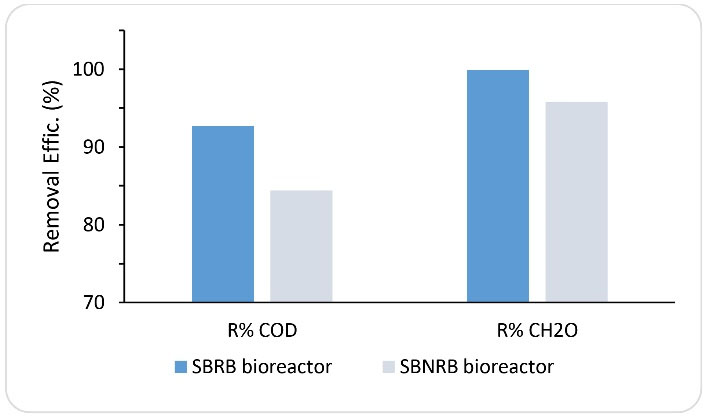
Figure 6.
Performance of Sequencing Batch Rotating Bed Bioreactor (SBRB Bioreactor) and Sequencing Batch non-rotating Bed Bioreactor (SBNRB Bioreactor) in COD and CH2O Removal (HRT=24 Hour, Inlet Concentration of CH2O =1000).
.
Performance of Sequencing Batch Rotating Bed Bioreactor (SBRB Bioreactor) and Sequencing Batch non-rotating Bed Bioreactor (SBNRB Bioreactor) in COD and CH2O Removal (HRT=24 Hour, Inlet Concentration of CH2O =1000).
3.5. The Influence of Hydrogen Peroxide Injection
As mentioned in section 3.3, at an inlet formaldehyde concentration of 1000 mg/L, the lowest performance of SBRB bioreactor was obtained at an HRT of 8 hours. Therefore, this operating condition was used to determine the effect ofhydrogen peroxide injection on the performance of SBRB bioreactor. The injection of hydrogen peroxide was performed at concentrations of 0, 0.1, 0.3, and 0.5 mM. Fig. 7 indicates the changes in the removal efficiency of COD and formaldehyde at the different concentrations of hydrogen peroxide injection. At the beginning of this phase, without injection of hydrogen peroxide, the removal rates of formaldehyde and COD were 95% and 57%, respectively. By adding hydrogen peroxide up to a concentration of 0.3 mM, the performance of the reactor was increased. In these conditions, the removal efficiency of formaldehyde and COD reached 99.2 % and 81%, respectively. However, the injection of hydrogen peroxide at a concentration of 0.5 mM disrupted the performance of the reactor. Therefore, by adding hydrogen peroxide under the optimal conditions (0.3 mM of hydrogen peroxide), an improvement (4% and 22%) in formaldehyde and COD removal efficiency was observed compared to the conditions before the injection of hydrogen peroxide (Fig. 8). As mentioned in all stages of this study, in addition to measuring the concentration of formaldehyde, the concentration of COD was also measured to determine the fate of formaldehyde as a result of biodegradation. Since the results obtained in all stages of the study showed that the percentage of formaldehyde removal was higher than the percentage of COD removal, it can be concluded that the formaldehyde removed by the biological process was not completely converted to CO2 and H2O2, but a fraction of the removed formaldehyde was converted to intermediate organic compounds. However, increasing COD removal efficiency with the injection of hydrogen peroxide can be considered a promising operation in the performance of a biological reactor. In fact, the greater the removal of COD, the greater the removal of formaldehyde-mediated organic compounds. The injection of the proper amount of hydrogen peroxide into the reactor can stimulate bacteria to produce more peroxidase and dehydrogenase enzymes. (18-20). Figs. 9a and 9b show the changes in peroxidase and dehydrogenase enzymes activity in SBRB bioreactor at different concentrations of hydrogen peroxide injection. As shown in Figs. 9a and 9b, the enzymatic activities of dehydrogenase and peroxidase increase with the addition of hydrogen peroxide and reach the highest amount at a hydrogen peroxide concentration of 0.3 mM. However, the enzymatic activity was decreased by increasing the amount of hydrogen peroxide injection to 0.5 mM. The decrease in enzymatic activity at a concentration of 0.5 mM is due to the inhibitory or toxicity effect of hydrogen peroxide on bacteria. Therefore, the addition of hydrogen peroxide should be done in an appropriate amount. Biostimulation of microorganisms with hydrogen peroxide can lead to the production of peroxidase. The results show that with increasing peroxidase activity, dehydrogenase activity also increases, which can be a strong reason for the high ability of bacteria against toxic compounds under conditions of high peroxidase secretion (14,17). The production of free radicals and the initiation of bond fission reactions in wastewater are among the capabilities of this enzyme (peroxidase), which subsequently cause the decomposition of organic compounds and provide the redox potential required for aerobic metabolism (21). Peroxidase activity involves the donation of electrons that attach to other substrates, breaking them into harmless compounds. Peroxidases have a common catalytic mechanism for the degradation of hydrogen peroxide (22).
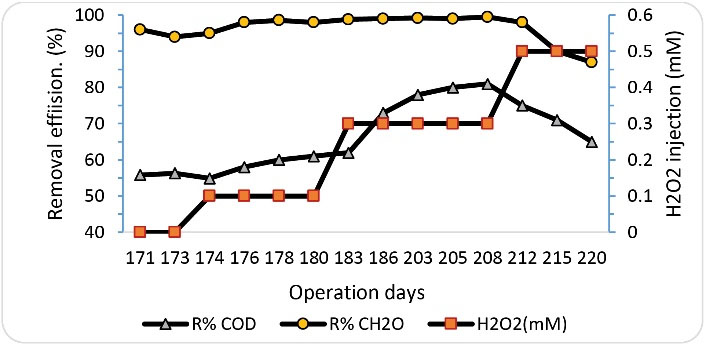
Figure 7.
The Effect of Various Amount of Hydrogen Peroxide Injection on Formaldehyde and COD Removal (HRT=8 Hour, Inlet Concentration of Formaldehyde = 1000 mg/L).
.
The Effect of Various Amount of Hydrogen Peroxide Injection on Formaldehyde and COD Removal (HRT=8 Hour, Inlet Concentration of Formaldehyde = 1000 mg/L).
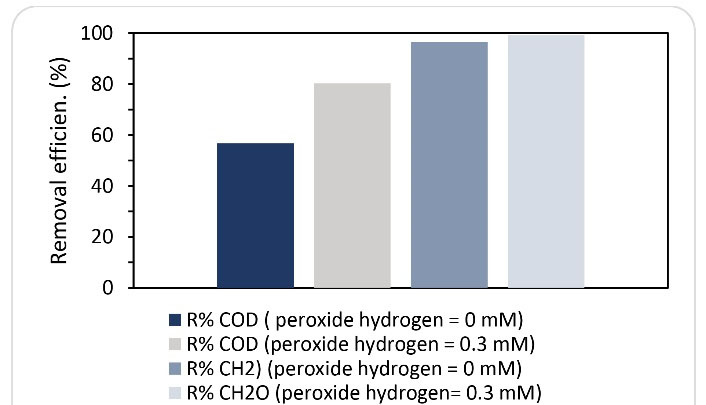
Figure 8.
Comparison of Reactor Performance with and without Injection of Hydrogen Peroxide on the Removal of Formaldehyde and COD (HRT= 8 Hours, Inlet Concentration of Formaldehyde = 1000 mg/L.
.
Comparison of Reactor Performance with and without Injection of Hydrogen Peroxide on the Removal of Formaldehyde and COD (HRT= 8 Hours, Inlet Concentration of Formaldehyde = 1000 mg/L.
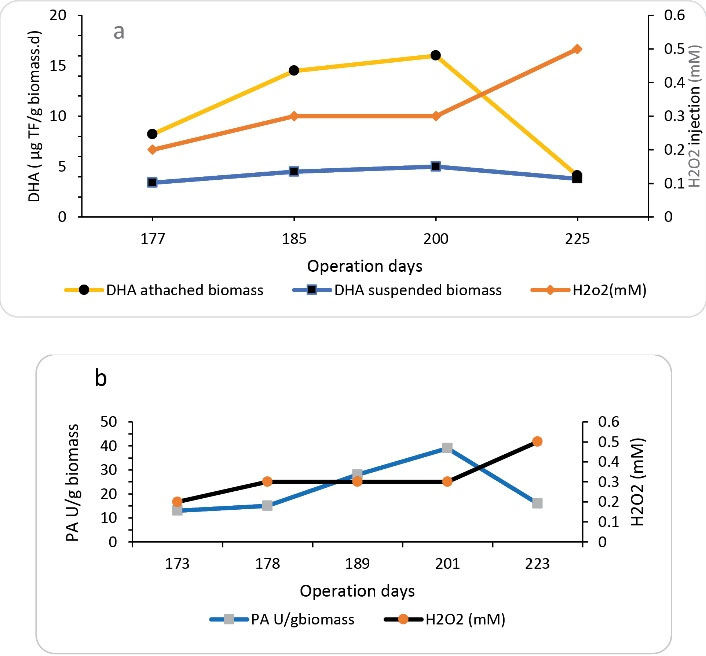
Figure 9.
Changes in Dehydrogenase and Peroxidase Enzyme Activity due to Various Amounts of Hydrogen Peroxide Injection (HRT=8 Hour, Inlet formaldehyde Concentration =1000 mg/L). Abbreviation: DHA, dehydrogenase.
.
Changes in Dehydrogenase and Peroxidase Enzyme Activity due to Various Amounts of Hydrogen Peroxide Injection (HRT=8 Hour, Inlet formaldehyde Concentration =1000 mg/L). Abbreviation: DHA, dehydrogenase.
3.6. Trend of Fluctuations in MLSS
The trend of fluctuations in MLSS during different phases of SRBR reactor operation is shown in Fig. 10. As shown in the figure during the start-up phase, which lasted 50 days, the amount of MLSS in the reactor gradually increased; in other words, from the 15th day onwards, the amount of attached MLSS in the reactor increased compared to the suspended MLSS. A further increase in the amount of attached MLSS can be related to the high surface area of the media, as well as the rotation of the bed in the reactor. In this phase, the average concentration of attached and suspended MLSS was found to be 2440 and 1770 mg/L, respectively. In this phase, the average total MLSS was 4214 mg/L. From the 51st day onwards, the SBRB reactor was gradually fed with a formaldehyde concentration of 500 mg/L to 1500 mg/L. In this phase, the concentration of attached MLSS increased but the concentration of suspended MLSS decreased slightly. At this stage, the average concentration of attached and suspended MLSS was measured to be 3100 and 2200 mg/L, respectively. However, in this phase, the average total concentration of MLSS was 5500 mg/L. In the next phase, i.e., from day 136 to day 170, the reactor was operated without rotating the basket containing the media. In this phase, the efficiency of the reactor in removing formaldehyde and COD reduced, and as shown in Fig. 8, the concentration of attached biomass decreased significantly but the concentration of the suspended biomass increased. The average of attached and suspended MLSS at this phase was found to be 1700 and 2720 mg/L, respectively. The non-rotation of the bed in this phase, less contact of the attached biomass with the substrate, and the reduction of mass transfer are the reasons for the significant reduction of the attached growth. From day 171 onwards, the SBRB reactor was operated by adding hydrogen peroxide. As shown in Fig. 10, at this stage, the amount of MLSS varied according to the dosage of hydrogen peroxide injected into the system. At a hydrogen peroxide concentration of 0.3 mM, the highest increase was observed in attached MLSS. At this stage, the average concentration of attached and suspended MLSS was found to be 4050 and 2300 mg/L, respectively. The mean total MLSS at this stage with a concentration of 6300 mg/L was the highest concentration of biomass in all stages of the study. However, in this phase, by adding 0.5 mM of hydrogen peroxide, the reactor performance was disrupted and the concentration of MLSS was significantly reduced. Increasing the enzymatic activity by adding the proper amount of hydrogen peroxide increases the rate of substrate removal and further growth of biomass in the reactor (14,22).
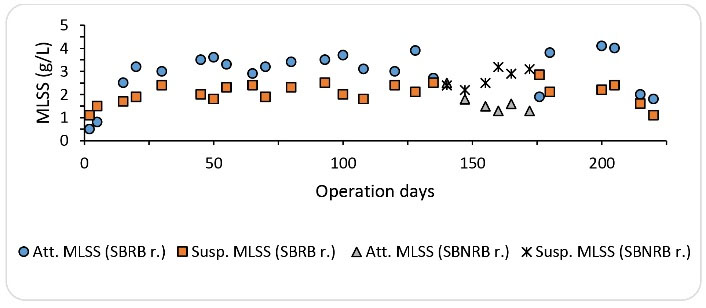
Figure 10.
Trends of Fluctuations of Suspended and Attached Biomass during the Operation Periods (Acclimation phase: days 1 to 50, Formaldehyde inlet concentration phase: days 51 to 93, Various HRTs: days 94 to 135, operation with non-rotating bed: days 136 to 172, Injection of H2O2: days 172 to 220).
.
Trends of Fluctuations of Suspended and Attached Biomass during the Operation Periods (Acclimation phase: days 1 to 50, Formaldehyde inlet concentration phase: days 51 to 93, Various HRTs: days 94 to 135, operation with non-rotating bed: days 136 to 172, Injection of H2O2: days 172 to 220).
4. Conclusion
In this study, the biodegradation of formaldehyde from wastewater was investigated using the SBRB bioreactor under long-term operation. The performance of the reactor with and without hydrogen peroxide injection was also investigated. The results revealed that the SBRB bioreactor is able to remove high concentrations of formaldehyde from wastewater with high efficiency. The use of this reactor with and without rotating the bed showed that the performance of the reactor was much better with rotating bed, especially for the removal of corresponding COD. In addition, the bioreactor with rotating bed (SBRB bioreactor) had higher stability and tolerance to applied shocks. The operation of the SBRB bioreactor with the injection of hydrogen peroxide indicated that high efficiencies can be obtained to remove formaldehyde and corresponding COD at a shorter HRT. The injection of hydrogen peroxide into the bioreactor in appropriate amounts increases the production of the peroxidase, leading to further breakdown of formaldehyde and other intermediate organic compounds. Finally, it can be concluded that the application of the SBRB bioreactor with and without hydrogen peroxide injection is capable of biodegrading high concentrations of formaldehyde. Therefore, this bioreactor may be used to remove other inhibitory organic compounds from wastewater.
Acknowledgment
This research with grant number 14655 was supported by the Vice-Chancellor of Research Affairs of Shahid Beheshti University of Medical Sciences, Tehran, Iran. Additionally, it was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (R.SBMU.PHNS.REC.1397.28). The authors would like to thank the Vice Chancellor of Research Affairs of Shahid Beheshti University of Medical Sciences.
Conflict of Interests
The authors declared that they have no conflict of interest.
References
- Moussavi G, Bagheri A, Khavanin A. The investigation of degradation and mineralization of high concentrations of formaldehyde in an electro-Fenton process combined with the biodegradation. J Hazard Mater 2012; 237-238:147-52. doi: 10.1016/j.jhazmat.2012.08.022 [Crossref] [ Google Scholar]
- Gong Y, Zhou X, Ma X, Chen J. Sustainable removal of formaldehyde using controllable water hyacinth. J Clean Prod 2018; 181:1-7. doi: 10.1016/j.jclepro.2018.01.220 [Crossref] [ Google Scholar]
- Moussavi G, Yazdanbakhsh A, Heidarizad M. The removal of formaldehyde from concentrated synthetic wastewater using O3/MgO/H2O2 process integrated with the biological treatment. J Hazard Mater 2009; 171(1-3):907-13. doi: 10.1016/j.jhazmat.2009.06.090 [Crossref] [ Google Scholar]
- Salthammer T. Formaldehyde sources, formaldehyde concentrations and air exchange rates in European housings. Build Environ 2019; 150:219-32. doi: 10.1016/j.buildenv.2018.12.042 [Crossref] [ Google Scholar]
- Yuan D, Tian L, Gu D, Shen X, Zhu L, Wu H. Fast and efficient oxidation of formaldehyde in wastewater via the Solar Thermal Electrochemical Process tuned by thermo-electrochemistry. J Clean Prod 2017; 156:310-6. doi: 10.1016/j.jclepro.2017.04.022 [Crossref] [ Google Scholar]
- US Environmental Protection Agency. Facts about Formaldehyde, Health Effects of Formaldehyde. Available from: https://www.epa.gov/formaldehyde/facts-about-formaldehyde#whatare.
- Qaderi F, Ayati B, Ganjidoust H. Role of moving bed biofilm reactor and sequencing batch reactor in biological degradation of formaldehyde wastewater. Iran J Environ Health Sci Eng 2011; 8(4):295-306. [ Google Scholar]
- Talaiekhozani A, Salari M, Talaei MR, Bagheri M, Eskandari Z. Formaldehyde removal from wastewater and air by using UV, ferrate(VI) and UV/ferrate(VI). J Environ Manage 2016; 184(Pt 2):204-9. doi: 10.1016/j.jenvman.2016.09.084 [Crossref] [ Google Scholar]
- Mei X, Guo Z, Liu J, Bi S, Li P, Wang Y. Treatment of formaldehyde wastewater by a membrane-aerated biofilm reactor (MABR): the degradation of formaldehyde in the presence of the cosubstrate methanol. Chem Eng J 2019; 372:673-83. doi: 10.1016/j.cej.2019.04.184 [Crossref] [ Google Scholar]
- Xing B, Chen H, Zhang X. Removal of organic phosphorus and formaldehyde in glyphosate wastewater by CWO and the lime-catalyzed formose reaction. Water Sci Technol 2017; 75(5-6):1390-8. doi: 10.2166/wst.2017.006 [Crossref] [ Google Scholar]
- Khodabakhshi A, Hatami V, Hemati S, Sadeghi M. Removal of formaldehyde from aqueous solutions by advanced oxidation processes: UV/S2O8 2-/Fe2+ and UV/S2O8 2-. Int J Environ Health Eng 2020; 9(1):20. doi: 10.4103/ijehe.ijehe_25_20 [Crossref] [ Google Scholar]
- Du L, Gao W, Li Z, Jiao W, Liu Y. Oxidative degradation of formaldehyde in wastewater by MgO/O3/H2O2 in a rotating packed bed. Chem Eng Process 2020; 155:108053. doi: 10.1016/j.cep.2020.108053 [Crossref] [ Google Scholar]
- Yazdanbakhsh AR, Eslami A, Najafi A. Performance of aerobic sequencing batch reactor (SBR) for formaldehyde removal from synthetic wastewater. Iran J Health Environ 2013; 6(2):233-42. [ Google Scholar]
- Moussavi G, Heidarizad M. The performance of SBR, SCR, and MSCR for simultaneous biodegradation of high concentrations of formaldehyde and ammonia. Sep Purif Technol 2011; 77(2):187-95. doi: 10.1016/j.seppur.2010.11.028 [Crossref] [ Google Scholar]
- Aghapour AA, Moussavi G, Yaghmaeian K. Investigating the performance of a novel cyclic rotating-bed biological reactor compared with a sequencing continuous-inflow reactor for biodegradation of catechol in wastewater. Bioresour Technol 2013; 138:369-72. doi: 10.1016/j.biortech.2013.03.133 [Crossref] [ Google Scholar]
- Nejad HM, Moussavi G. Advanced biodegradation process of atrazine in the peroxidase-mediated sequencing batch reactor (SBR) and moving-bed SBR (MSBR): mineralization and detoxification. J Environ Health Sci Eng 2020; 18(2):433-9. doi: 10.1007/s40201-020-00471-2 [Crossref] [ Google Scholar]
- Pourakbar M, Moussavi G, Yaghmaeian K. Enhanced biodegradation of phenol in a novel cyclic activated sludge integrated with a rotating bed bioreactor in anoxic and peroxidase-mediated conditions. RSC Adv 2018; 8(12):6293-305. doi: 10.1039/c7ra12997a [Crossref] [ Google Scholar]
- Shekoohiyan S, Moussavi G, Naddafi K. The peroxidase-mediated biodegradation of petroleum hydrocarbons in a H2O2-induced SBR using in-situ production of peroxidase: Biodegradation experiments and bacterial identification. J Hazard Mater 2016; 313:170-8. doi: 10.1016/j.jhazmat.2016.03.081 [Crossref] [ Google Scholar]
- Moussavi G, Abbaszadeh Haddad F. Bacterial peroxidase-mediated enhanced biodegradation and mineralization of bisphenol A in a batch bioreactor. Chemosphere 2019; 222:549-55. doi: 10.1016/j.chemosphere.2019.01.190 [Crossref] [ Google Scholar]
- Mielgo I, Moreira MT, Feijoo G, Lema JM. Biodegradation of a polymeric dye in a pulsed bed bioreactor by immobilised phanerochaete chrysosporium. Water Res 2002; 36(7):1896-901. doi: 10.1016/s0043-1354(01)00384-0 [Crossref] [ Google Scholar]
- Shekoohiyan S, Moussavi G, Naddafi K. The peroxidase-mediated biodegradation of petroleum hydrocarbons in a H2O2-induced SBR using in-situ production of peroxidase: biodegradation experiments and bacterial identification. J Hazard Mater 2016; 313:170-8. doi: 10.1016/j.jhazmat.2016.03.081 [Crossref] [ Google Scholar]
- Bansal N, Kanwar SS. Peroxidase(s) in environment protection. ScientificWorldJournal 2013; 2013:714639. doi: 10.1155/2013/714639 [Crossref] [ Google Scholar]
- van Bloois E, Torres Pazmiño DE, Winter RT, Fraaije MW. A robust and extracellular heme-containing peroxidase from Thermobifida fusca as prototype of a bacterial peroxidase superfamily. Appl Microbiol Biotechnol 2010; 86(5):1419-30. doi: 10.1007/s00253-009-2369-x [Crossref] [ Google Scholar]
- Alneyadi AH, Ashraf SS. Differential enzymatic degradation of thiazole pollutants by two different peroxidases–a comparative study. Chem Eng J 2016; 303:529-38. doi: 10.1016/j.cej.2016.06.017 [Crossref] [ Google Scholar]
- Alneyadi AH, Shah I, AbuQamar SF, Ashraf SS. Differential degradation and detoxification of an aromatic pollutant by two different peroxidases. Biomolecules 2017; 7(1):31. doi: 10.3390/biom7010031 [Crossref] [ Google Scholar]
- Nash T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J 1953; 55(3):416-21. doi: 10.1042/bj0550416 [Crossref] [ Google Scholar]
- Baird RB, Eaton AD, Rice EW, Bridgewater L. Standard Methods for the Examination of Water and Wastewater. Vol 23. Washington, DC: American Public Health Association (APHA); 2017.
- Pereira NS, Zaiat M. Degradation of formaldehyde in anaerobic sequencing batch biofilm reactor (ASBBR). J Hazard Mater 2009; 163(2-3):777-82. doi: 10.1016/j.jhazmat.2008.07.028 [Crossref] [ Google Scholar]
- Moussavi G, Heidarizad M. Biodegradation of mixture of phenol and formaldehyde in wastewater using a single-basin MSCR process. J Biotechnol 2010; 150(2):240-5. doi: 10.1016/j.jbiotec.2010.08.012 [Crossref] [ Google Scholar]
- Bolonhesi I, Andreani CL, Theodoro JD, Fazolo A, Lopes DD. Formaldehyde biodegradation in an ASBR-SBR system: an effective treatment solution for furniture industry painting booth wastewater. Int J Environ Sci Technol 2022; 19(4):3075-86. doi: 10.1007/s13762-021-03276-4 [Crossref] [ Google Scholar]
- Wang ZH, Wei HB, Jia ZY, Lu YM, Liu X, Zou P. Activated sludge process for treatment of formaldehyde wastewater. China Water & Wastewater 2009; 25(1):86-7. [ Google Scholar]
- Farzadkia M, Jorfi S, Estebar M. Treatment of synthetic wastewaters contaminated with formaldehyde using an anaerobic sequencing batch biofilm reactor. Journal of School of Public Health & Institute of Public Health Research 2010; 8(1):31-40. [ Google Scholar]
- Jaafarzadeh N, Jorfi S, Yaghmaeian K, Talaie AR, Hashempour Y. Effects of biofilm in improvement of activated sludge efficiency for treatment of industrial effluents containing formaldehyde. Koomesh 2011; 12(2):215-21. [ Google Scholar]