Avicenna J Environ Health Eng. 7(2):97-108.
doi: 10.34172/ajehe.2020.15
Original Article
Isothermal, Kinetic, and Thermodynamic Studies on the Adsorption of Molybdenum by a Nanostructured Magnetic Material
Elnaz Shargh 1, Hossein Sid Kalal 2, *  , Zahra Shiri-Yekta 2, Seyed Ebrahim Mousavi 3, Mohammad Reza Almasian 2, Mohmmad Tagiof 2, Hassan Hoveidi 1
, Zahra Shiri-Yekta 2, Seyed Ebrahim Mousavi 3, Mohammad Reza Almasian 2, Mohmmad Tagiof 2, Hassan Hoveidi 1
Author information:
1Department of Environmental Management, Faculty of the Environment, University of Tehran, Tehran, Iran
2Nuclear Fuel Research School, Nuclear Science and Technology Research Institute, AEOI, P.O. Box 11365-3486, Tehran, Iran
3School of Chemical Engineering, College of Engineering, University of Tehran, Tehran, Iran
Abstract
In this study, the magnetic 3-(trimethoxysilyl) propyl methacrylate (TMSPMA) – poly (4-vinylpyridine) (P4VP) was synthesized and characterized. Removal of Molybdenum (Mo) from aqueous solutions using prepared material as nanosorbent was investigated. The magnetic P4VP was prepared by copolymerization of P4VP with TMSPMA. The prepared adsorbent was characterized by various techniques including the X-ray powder diffraction (XRD), scanning electron microscopy (SEM), and Fourier-transform infrared spectroscopy (FTIR). The batch adsorption technique was applied and the effect of several important parameters such as pH of the aqueous solution, adsorbent dose, initial Mo(VI) concentration, contact time, and temperature was evaluated. Desorption behavior of Mo(VI) and the effect of foreign ions (Cd2+, Ca2+, Co2+, Fe3+, Ba2+ and Pt4+) in real samples were also investigated. Co (II) and Pt (IV) had a greater impact on the adsorption process than other foreign ions. The maximum capacity for Mo(VI) adsorption on the prepared adsorbent was 4.87 mg/g, which was obtained at a temperature of 40°C with an initial concentration of 10 mg/L of Mo(VI). The adsorption isotherms were best fitted with the Weber Van Vliet isotherm model. The kinetic data were fitted well with the pseudo-second-order equation with a high correlation coefficient (R2 > 0.99). Based on the negative standard Gibbs free energy change (ΔG° < 0) and the positive standard enthalpy change (ΔH° > 0), it was found that the adsorption was an endothermic and a spontaneous process in nature.
Keywords: Molybdenum removal, Batch adsorption, 3-(trimethoxysilyl) propyl methacrylate, Poly (4-vinylpyridine), Magnetic nanosorbent
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
Molybdenum (Mo) has important applications for different fields. Mo can be used as an alloying agent in cast iron, steels, reactor vessels, and special batteries due to its various properties. Mo is also applied in solid lubricants, automotive industry, plastics and rubber industry, aircraft engines, glass industry, pigments (for paints, coatings and inks), as well as in electronics industry. There are high concentrations (>5 ppm) of molybdate (MoO4 2-) in the environment, which have harmful effects on humans, vegetables, and Animals (1). An examination of the environmental hazards of Mo showed that it is generally a hazardous substance and if swallowed, it can cause severe poisoning. Symptoms of acute poisoning include severe irritation of the gastrointestinal tract with diarrhea, coma, and death from heart failure. It affects the nervous and pulmonary systems in much the same way as it affects the respiratory tract, and it can also cause skin allergies and severe damage to the eyes. This element is flammable in powder form (2). Mo is the most concentrated trace metal in seawater due to its high stability and weak adsorption. Given the above-mentioned reasons, the separation of Mo is mandatory. Molybdenum-99 (99Mo) is a radionuclide used in the nuclear fields and medical practices (2-4). Mo belongs to the fission products and is contained in significant concentrations in highly radioactive waste. Due to the complex chemical properties of Mo, soluble and insoluble complexes are formed. In particular, several problems occur in the concentration, storage, and solidification of high-quality waste (5). The proposed methods for removing molybdate (molybdate includes Mo(VI) ion) from aqueous solutions have been described with various adsorbents, namely carbon cloths (6,7), aluminum oxides (8), pyrite (9,10), natrolite (11), goethite (9), and iron oxide gel (12). Nanomaterials are kinds of materials that have a particle size of 100 nm or less with particular specifications (13). Many researchers have reported the application of different nanosized adsorbents such as nanoparticles (NPs) and nanotubes (14). Recently, the preparation of magnetic nanoparticles (MNPs) has received a lot of attention because of its various technological applications in water purification, photocatalysis and other separation processes (such as extraction bioseparation (15-17), and separation of different ions (17-32). The MNPs are effective adsorbents due to their large specific surface area, low toxicity, and superparamagnetic behavior. Due to their magnetic properties, they can be used for effective separation in a short time using an external magnetic field (33). Chemical modification and surface functionalization of adsorbents make it possible to use them in various physical, chemical, and biological conditions (34). The Fe3O4 MNPs have received a great deal of attention in various academic and technological applications because of their inherent properties (35-37). The present study aimed to synthesize the magnetic poly (4-vinylpyridine) (P4VP), which was prepared by copolymerization of P4VP with 3-(trimethoxysilyl) propyl methacrylate (TMSPMA). The physical and chemical characteristics of the synthesized adsorbent were specified. The adsorbent efficiency in removing Mo(VI) ions from aqueous solutions was investigated. Several important parameters were evaluated and optimized. The kinetic, isotherm, thermodynamic, and error investigations were also performed.
2. Materials and Methods
Sodium molybdate dihydrate (Na2MoO4·2H2O) was prepared by diluting a 1000 mg/L Mo(VI) solution. All chemicals and reagents applied in this study were purchased from Merck Company (Merck, Darmstadt, Germany) and met the analytical standard. All solutions were prepared with deionized and double-distilled water. The prepared adsorbent was characterized by several techniques including Fourier-transform infrared spectroscopy (FTIR) (Shimadzo, FT-IR4300 spectrophotometer in the range of 400-4000 cm-1), X-ray powder diffraction (X’pert, Philips, Holland in the 2θ range of 5-100°), and scanning electron microscopy (SEM) (Hitachi S-4160 scanning electron microscope). The concentration of metal ions was determined by inductively coupled plasma (ICP) (Varian liberty 150 XL).
2.1. Synthesis and Functionalization of Fe3O4 NPs
First step: FeCl2.4H2O (3.32 g, 0.05 mmol) and FeCl3.6H2O (5.4 g, 0.06 mmol) were added to a water solution (80 mL). Ammonia solution (25 wt %) was added to the mixture to adjust the pH value to 9. The resultant mixture was kept at room temperature for 12 hours. Then the NPs were disconnected from the solution with a permanent magnetic, and washed several times with water until the pH value reached 7. Finally, the Fe3O4 NPs were dried in a vacuum oven at 80°C for 24 hours.
Second step: The purified Fe3O4 NPs were added to 45 mL deionized water. Then, 2.0 mL of TMSPMA was added to the Fe3O4 NPs solution under mechanical agitation at room temperature. Surface silanization of Fe3O4 MNPs was performed for 24 hours. Then, Surface modified Fe3O4 MNPs were gathered and rinsed with deionized water several times by a magnet (Fig. 1).

Figure 1.
Adsorbent Appearance Before and After Separation With the Magnetic Field (Magnet).
.
Adsorbent Appearance Before and After Separation With the Magnetic Field (Magnet).
Third step: TMSPMA-functionalized Fe3O4 (2.5 g) and 500 mL of Toluene were added to the 1-L flask and then the mixture was subjected to ultrasound for 10 minutes. After dispersing, 7.0 g of 4-vinylpyridine was monomerically dissolved in the mixture. Then 0.07 g of AIBN was added to the piston and the reaction container was warmed to 70°C for initiating solution polymerization under N2 atmosphere and agitation for 24 hours. After the polymerization step, the resolution was rinsed once with acetone, and the free P4VP was extracted in Soxhlet apparatus with hot methanol for 36 hours. Finally, P4VP-grafted Fe3O4 MNPs were prepared. The formation of functionalized NPs is shown in Fig. 2.
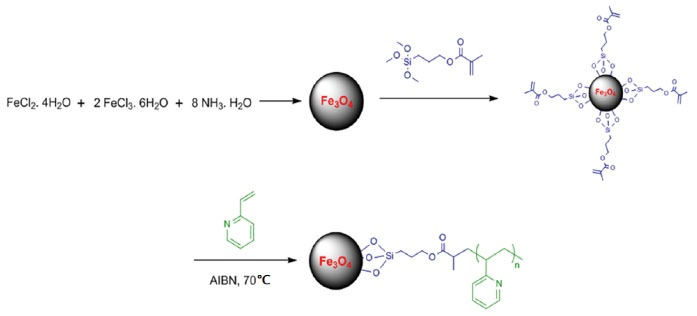
Figure 2.
Schematic of the Synthesis of Functional Fe3O4 Nanoparticles.
.
Schematic of the Synthesis of Functional Fe3O4 Nanoparticles.
2.2. Batch Sorption Studies
Batch sorption experiments were performed by setting the precise quantity of Fe3O4/SiO2/P4VP nanosorbent in 25 mL solution under different situations. The primary pH value of the aqueous solution was set with 0.1 M HNO3 and 0.1 M NaOH or 0.1 M NH3. The effect of the most important parameters in different ranges (these ranges were chosen based on similar studies) was investigated as follows:
-
The aqueous solution pH values were varied between 1.5 to 9, at 25°C and time of 210 minutes.
-
The adsorbent dose values were varied between 0.01 and 0.1 g, at a temperature of 25°C, in 210 minutes contact time, and with optimum pH.
-
The metal concentrations were varied between 0.5–3 mg/L, at a temperature of 25°C, in 210 minutes contact time, and with optimum pH and adsorbent dose.
-
The influence of contact time was examined by changing the time from 5 to 120 minutes, at 25°C and with selected pH value, adsorbent dosage, and initial concentration.
-
The impact of temperature was investigated in the range of 20–40°C, in 210 minutes, and with an optimum pH, adsorbent dose and initial concentration.
The percentage of removal efficiency (1), desorption (2) and the adsorption capacity (3) for Mo(VI) ions were determined based on the following formulas:
(1)
(2)
(3)
Where C0 and Ce (mg/L) are the initial and equilibrium liquid-phase concentrations of metal ions, respectively. The value qe (mg/ g) is the sorption capacity. Values md and ma are the desorption and adsorption of ions from the adsorbent surface (mg), respectively. V (L) is the volume of the solution and m (g) is the weight of dried used adsorbent.
3. Results and Discussion
3.1. Adsorbent Characterization
3.1.2. FT-IR Spectroscopy
To ascertain the presence of SiO2 and P4VP on Fe3O4 MNPs, FTIR spectra were provided from Fe3O4 MNPs, Fe3O4/SiO2, and Fe3O4/SiO2/P4VP (Fig. 3). From the FTIR spectra shown in Fig. 3 (curve a), it is evident that the characteristic peak of Fe3O4 MNPs appeared at 588 cm−1. This band was shifted to a high wave number compared to the Fe-O bond peak of bulk magnetite at 570 cm−1 due to nanoparticle size (38). The Si–O–Si bond’s asymmetric stretching vibration at 1100 cm−1 and symmetric stretching vibration at around 800 cm−1 appear in Fe3O4/SiO2 and Fe3O4/SiO2/P4VP spectra (Fig. 3 (curve b and c)), which indicates that the silica has successfully coated on the surface of Fe3O4 NPs.
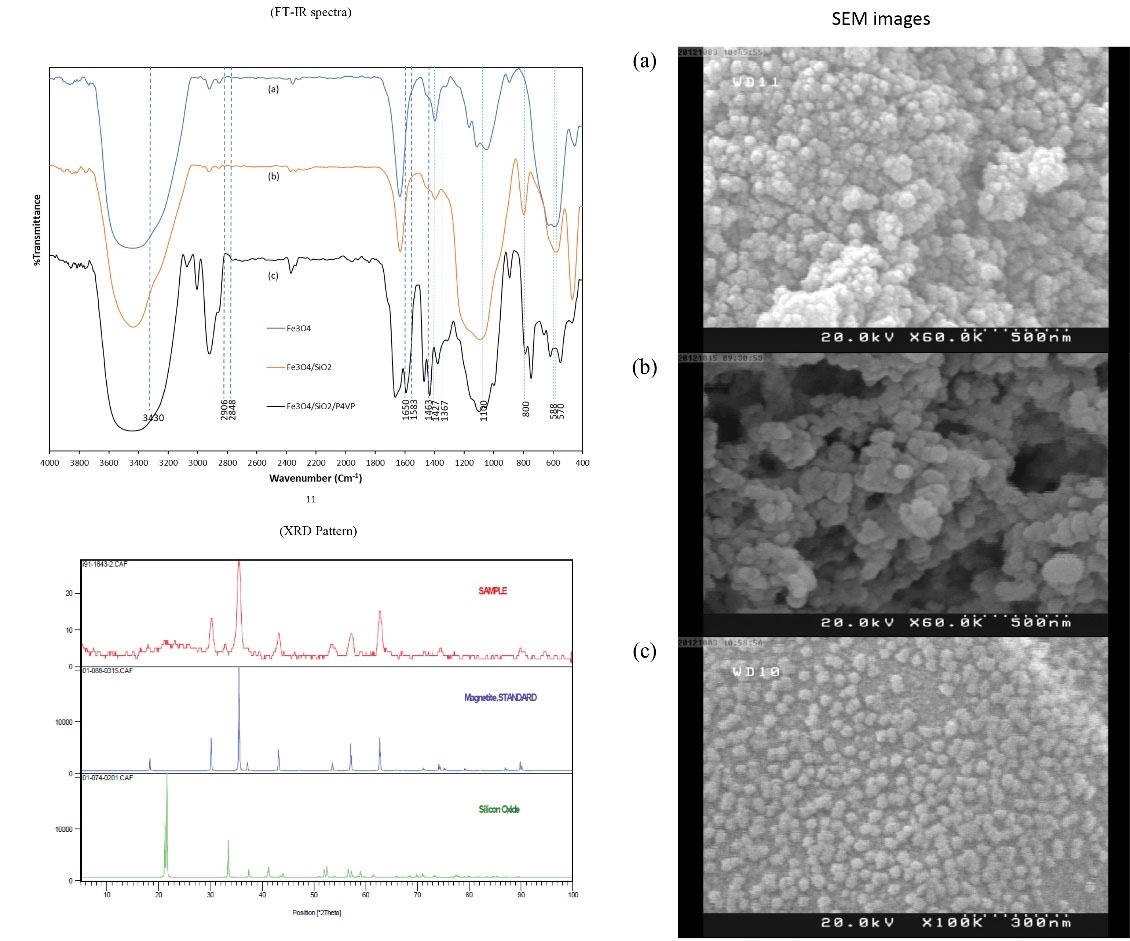
Figure 3.
FT-IR Spectra of Fe3O4, Fe3O4/SiO2, and Fe3O4/SiO2/P4VP, XRD Pattern of Fe3O4
Nanoparticles, SiO2 and Fe3O4/SiO2, and SEM Images of (a) Base Magnetite Particles,
(b) Fe3O4/SiO2 Particles and (c) Fe3O4/SiO2/P4VP Particles
.
FT-IR Spectra of Fe3O4, Fe3O4/SiO2, and Fe3O4/SiO2/P4VP, XRD Pattern of Fe3O4
Nanoparticles, SiO2 and Fe3O4/SiO2, and SEM Images of (a) Base Magnetite Particles,
(b) Fe3O4/SiO2 Particles and (c) Fe3O4/SiO2/P4VP Particles
Moreover, the peaks around 1583, 1463, and 1367 cm−1 which occurred in Fe3O4/SiO2/P4VP spectra, could be assigned as features of pyridine cycle and amine in the P4VP molecules. The absorption bands at about 3430 and 1650 cm−1 in all the spectra mainly originate from the –OH vibrations in H2O. Based on the presence of bands in the range of 2848–2906 cm-1 and 1427 cm-1, which correspond to the -CH2 stretching vibration in P4VP / Fe3O4 / SiO2 FTIR spectrum (black line in FTIR spectra), one can deduce that the P4VP molecules are bonded to the surface of Fe3O4 / SiO2.
3.1.3. XRD Pattern
Fig. 1 shows the X-ray diffraction result of the base magnetite particles. The result revealed diffraction peaks at 2Ɵ = 30.13, 35.54, 43.11, 53.73, 57.30 and 62.77, which are characteristic peaks of magnetite (Fe3O4) crystals (39). The average diameter which can be evaluated from the Scherrer equation (D = 0.9λ / β cos θ, where D is the average diameter, λ is the x-ray wavelength, β is the full width at wide half maximum and θ is the x-ray diffraction angel), is 42.4 nm. The XRD pattern of Fe3O4/SiO2 NPs (Fig. 3) revealed that the binding and TMSPMA did not cause any measurable change in the phase property of Fe3O4 cores. This could be attributed to the fact that the binding and TMSPMA occurred only on the surface of the Fe3O4 cores to form a core-shell structure.
3.1.4. Scanning Electron Microscopy
For obtaining more direct information on particle size and morphology, the SEM micrograph of base magnetite particles, Fe3O4/SiO2 and Fe3O4/SiO2/P4VP were provided. SEM photographs showed that the silanized MNPs (Fig. 3b) were roughly spherical in shape, and the average size of these particles was very close to the average size of base magnetite particles (Fig. 3a). The size distribution was 35.1–40.6 nm in base magnetite particles which almost matches the calculated value by the Scherrer equation. Fig. 3b, c represents the SEM images of Fe3O4/SiO2 and Fe3O4/SiO2/P4VP. The morphology and size almost maintained the original state (32.8 and 36 nm, respectively).
3.2. Adsorption Investigations
3.2.1. Effect of pH
The pH of the solution is an essential agent that has a great impact on the adsorption of metal ions. This is strikingly due to the difference in chemical characteristics of the sorbent, sorbate, and the ionization of dissolved ions, which can change the kinetic and equilibrium properties of the sorption process (40). Batch sorption experiments of Mo(VI) were performed by putting 0.01 g of Fe3O4/SiO2/P4VP nanosorbent in 25 mL of the metal solutions (2 mg/L) for 210 minutes. The pH value of the solutions was regulated with 0.1 M HNO3 or 0.1 M NaOH. The pH value was evaluated in the range of 1.5 to 9. The adsorption efficiency of Mo(VI) on the adsorbent as the purpose of initial solution pH (1.5–9) is shown in Fig. 4A. At a pH value of 2.5 and minor quantities, there was a powerful condition to develop a chelate complex with the amine combinations of the sorbent between H+ and Mo (VI) ions. This was due to the condition in which by reducing the pH, the protonation percentage of the amine combinations increased and, conversely, the number of active sorption sites for the metal ions adsorption decreased. By increasing the pH from 2.5 to 3.5, the positive charge density of the sorption reduced (due to the decrement in H+ concentration), and the active positions of the sorbent easily absorbed the metal ions. The adsorption efficiency for Mo(VI) was obtained with 89.8% (at pH = 3.5). At pH values above 3.5, the metal ions precipitated out as metal hydroxide due to the high concentration of OH anions. Therefore, the optimal pH range of 3.5 was chosen.
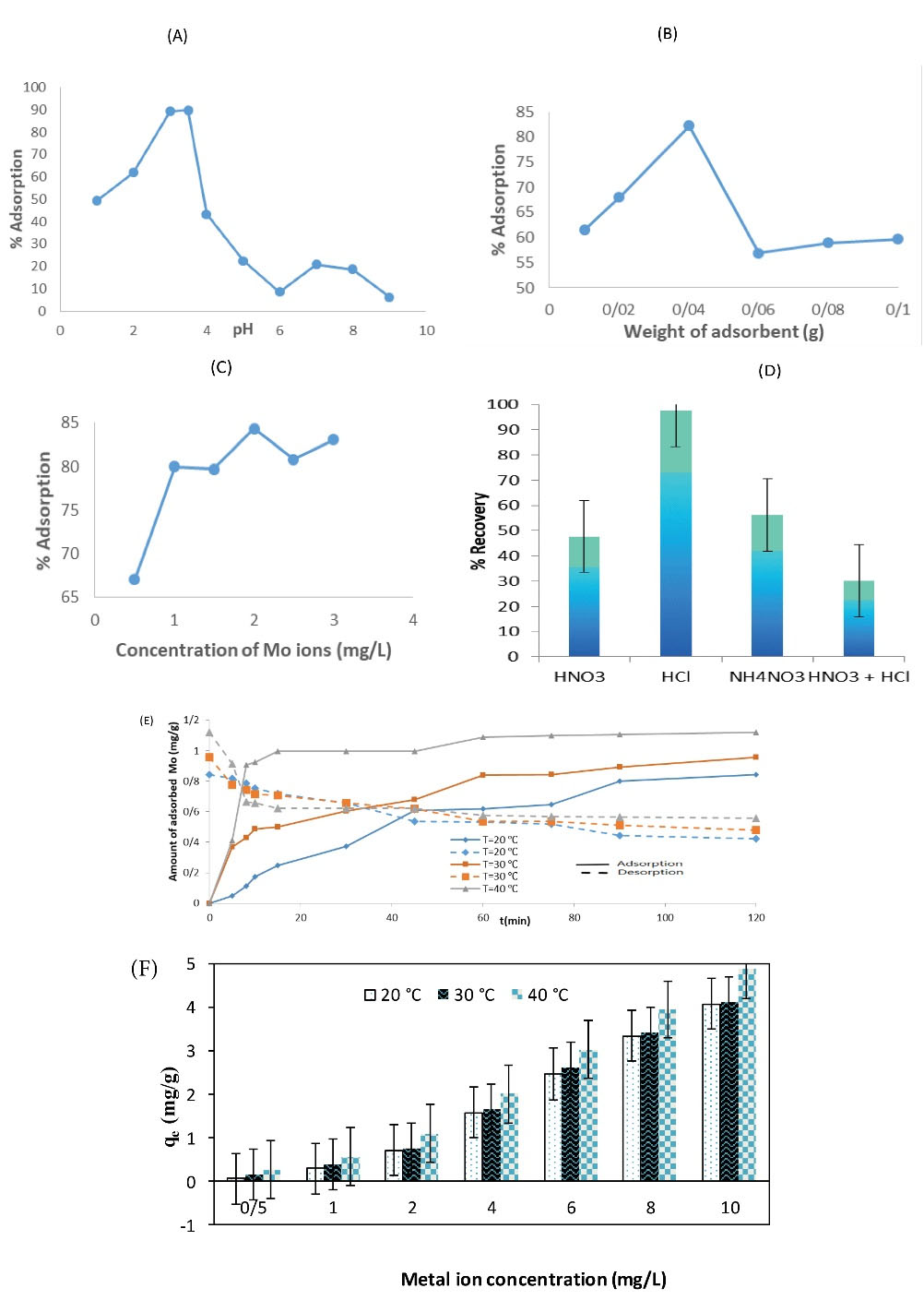
Figure 4.
(A) Effect of pH, (B) Sorbent Dosage, and (C) Initial Concentration of Mo(VI) on the Sorption of Mo(VI) Ions by Adsorbent, (D) Desorption Results of Mo(VI) from Adsorbent Surface at Different Solvents, (E) Effect of Contact time at Different Temperatures on the Adsorption and Desorption Processes, (F) Effect of Temperature on the Adsorption of Mo(VI) Onto the Adsorbent.
.
(A) Effect of pH, (B) Sorbent Dosage, and (C) Initial Concentration of Mo(VI) on the Sorption of Mo(VI) Ions by Adsorbent, (D) Desorption Results of Mo(VI) from Adsorbent Surface at Different Solvents, (E) Effect of Contact time at Different Temperatures on the Adsorption and Desorption Processes, (F) Effect of Temperature on the Adsorption of Mo(VI) Onto the Adsorbent.
3.2.2. Effect of Adsorbent Dose
The effect of the adsorbent dose on the amount of Mo (VI) sorption is shown in Fig. 4B. The sorption of Mo(VI) by the synthesized material (Fe3O4/SiO2/P4VP) was examined by changing the mass of sorbent in solutions, while keeping other parameters constant. By increasing the sorbent amount from 0.01 to 0.04 g, the adsorption efficiency was increased from 61.5 to 82.3%; but by re-rising it from 0.04 to 0.1 g, the adsorption efficiency was decreased. By increasing the sorption dose with a uniform metal ion concentration, the sorption sites are not saturated completely. At higher ratios of the mass of the adsorbent to concentration of metal ions, there was a surface sorption causing the metal ion concentration to decrease again when the ratio was reduced. This phenomenon is because of the fact that a specific quantity of sorbate can be separated by a solid enrichment of the adsorbent. The metal ions adsorbed causes a decrease in dose of the sorbent, which is due to a decrease in the concentration gradient between the concentrations of metal ions in the solution and on the surface of the sorbent. In addition, this reduction can be attributed to a partial covering or aggregation of sorption sites, which leads to a reduction in the total accessible sorbent surface for the metal ions and to an extension of the path of propagation (41,42). Finally, the best adsorbent dose was determined to be 0.04 g.
3.2.3. Effect of Initial Concentration
The effect of initial concentration (C0) on the adsorption characteristics was intensively investigated for Fe3O4/SiO2/P4VP by varying C0 of Mo(VI) from 0.5 to 3 mg/L at 25°C. The remaining conditions stayed fixed (no change) over the course of the tests. The results are shown in Fig. 4C. Under optimum conditions, the adsorption efficiency of Mo(VI) increased with the expansion of the initial Mo(VI) concentration from 67% to 83.1%. The phenomenon can be attributed to the fact that the total available adsorption sites are not entirely filled with a fixed adsorbent dose, which leads to an enrichment of the adsorption percentage of adsorbate. Eventually, 2 mg/L was selected as optimum C0 to continue the experiments.
3.2.4. Desorption Study
Four aqueous solutions (each 25 mL) containing Mo(VI) ions (2 mg/L) were magnetically stirred with 0.04 g of the Fe3O4/SiO2/P4VP particles, at 25°C and for 210 minutes. The uptake amount was 84.5% for Mo(VI) ions. The loaded adsorbents were filtered-off and then were contacted with 10 mL of HNO3, HCI, NH4NO3, and a mixture of HNO3 and HCI (1 M), separately. It was observed that the adsorbed ions were desorbed quantitatively using HCI (Fig. 4D). Then, to determine the optimal concentration of HCl, the used adsorbent was transferred to 10 mL of HCl in the concentration area of 0.5–5 M. The concentration of Mo(VI) was evaluated as previously mentioned. The desorption course was also followed to extract M0(VI) ions from wastewater and adsorbent. According to the results shown in Table 1, HCI 1 M Mo(VI) is suitable for recovery.
Table 1.
Desorption Results of Mo(VI) from Fe3O4/SiO2/P4VP Surface With HCl as Stripping Agent at Different Concentrations
|
HCI Concentration (M)
|
Recovery (%)
|
| 0.5 |
45.65 |
| 1 |
98.0 |
| 2 |
53.0 |
| 3 |
45.45 |
| 4 |
58.35 |
| 5 |
47.85 |
3.2.5. Effect of Contact Time and Temperature
The variations of Mo(VI) sorption amount in relation to the contact time in various temperatures (20, 30 and 40°C) were investigated (Fig. 4E). The other parameters stayed constant during the experiments. As it can be stated, the sorption of the Mo(VI) ions occurs in two stages: the first stage involves an external diffusion that follows the introduction of the metal ions into the outer surface of the adsorbent, which is a quick step (rapid phase). The second stage comprises a pore diffusion which follows the sorption of the metal ions on the inner surface of the adsorbent (drag phase). It was evident that the amount of Mo(VI) adsorbed was increased by an increase in the temperature. For Mo ions, the sorption rate was immediate within the first minutes (10 minutes), and this rate gradually decreased as it reached equilibrium. It was concluded that the sorption of Mo(VI) species onto the Fe3O4/SiO2/P4VP sorbent was an endothermic process. Ultimately, sorption is inherently exothermic and diffusion is an endothermic process. With an increase in the amount of adsorption due to rising temperatures, it was concluded that the role of the diffusion process had been more significant than the sorption progress. Under corresponding initial Mo(VI) concentrations from 0.5 to 10 mg/L, the adsorption capacity of Mo(VI) increased with an increase in temperature from 20 to 40°C. The temperature effect on the sorption capacity at the equilibrium state is demonstrated in the Table 2 and Fig. 4F.
Table 2.
Equilibrium Results With Mo(VI) Ions Initial Concentrations From 0.5 to 10 mg/L and Temperatures 293, 303 and 313 k
|
C
0
(mg/L)
|
T (K)
|
|
293
|
303
|
313
|
|
C
e
(mg/L)
|
Kd
|
q (mg/g)
|
C
e
(mg/L)
|
Kd
|
q (mg/g)
|
C
e
(mg/L)
|
Kd
|
q (mg/g)
|
| 0.5 |
0.404 |
0.216 |
0.06 |
0.261 |
1.110 |
0.149 |
0.075 |
5.130 |
0.266 |
| 1 |
0.541 |
0.702 |
0.287 |
0.400 |
2.222 |
0.375 |
0.111 |
6.538 |
0.556 |
| 2 |
0.853 |
1.075 |
0.717 |
0.783 |
2.467 |
0.760 |
0.256 |
5.366 |
1.090 |
| 4 |
1.120 |
2.162 |
1.800 |
1.374 |
2.735 |
1.641 |
0.784 |
3.409 |
2.010 |
| 6 |
1.412 |
2.784 |
2.866 |
1.828 |
3.142 |
2.608 |
1.165 |
3.519 |
3.022 |
| 8 |
1.701 |
3.114 |
3.937 |
2.520 |
3.111 |
3.425 |
1.688 |
3.108 |
3.945 |
| 10 |
2.369 |
2.654 |
4.769 |
3.510 |
2.761 |
4.056 |
2.210 |
2.866 |
4.869 |
3.2.6. Sorption Kinetics
Kinetics of the adsorption process is extremely important and remarkable for modeling and designing the sorption developed in the industries, as well as for describing the adsorption rate of dissolved substances, and it determines the time required for the adsorption reaction. Therefore, the kinetics of the removal of Mo(VI) ions was determined in this study in order for determining the sorption behavior of Fe3O4/SiO2/P4VP. Various kinetic models were considered to describe the experimental data obtained from the experiments, and to clarify the development of the kinetic sorption (Table 3). The pseudo-first order, pseudo-second order, and intraparticle diffusion types were all analyzed to describe the kinetic data obtained from the experiments. The parameters including rate constants ( k1, k2 ) and correlation coefficients were computed; the calculated parameters are shown in Table 4 and Fig. 5. The correlation coefficient values of the pseudo-first-order model were lower than the pseudo-second-order model. As can be seen in Table 4 below, the pseudo-second order model belongs to the kinetic data and describes the data more consistently due to the high value of the correlation coefficient (R2 > 0.99). The temperature effect on the adsorption rate constant was determined using the Arrhenius-type relationship (Table 3). The k0 of Ea were 7.9×1012 g/mg.min and 82.5497 kJ/mol with a C0 of 2.0 mg/L. The magnitude of activation energy showed a type of adsorption that was physical or chemical. Achieving activation energy of 5–40 kJ/mol coincides with physisorption mechanism, while achieving activation energy of 40-800 kJ/mol coincides with chemisorption mechanism(32). The activation energy (Ea) in this study was 82.5497 kJ/mol, which is associated with chemisorption and has a higher potential.
Table 3.
Isotherms, Kinetics, and Other Equations Used in This Study
| Models / Equations |
Parameters |
| Generalized model |
|
|
qe (mg/ g): equilibrium adsorption capacity |
| qm (mg/ g): maximum adsorption capacity |
Ce (mg/ L): equilibrium adsorbate concentration in solution
ng: generalized equation exponent |
| Kg(mg/ L): generalized constant |
| Fritz Schlunder model |
|
|
|
Kf1 (mg/g)(mg/L) nf: fritz schlunder constant |
| Kf2 (mg/ L) mf : Fritz Schlunder constant |
nf: fritz schlunder equation exponent
mf: fritz schlunder equation exponent |
| Weber Van Vliet model |
|
|
|
Kw : weber van vliet constant |
| nw: weber van vliet equation exponent |
| mw: weber van vliet equation exponent |
| lw: weber van vliet equation exponent |
| Pseudo-first-order kinetic model |
|
|
|
qt (mg/L): amount of adsorbate adsorbed at time t |
| k1 (min-1): pseudo-first-order rate constant |
| Pseudo- second order kinetic model |
|
|
|
k2 (g mg.min-1): pseudo-second-order rate constant
t (min): time |
| Intraparticle diffusion model |
|
|
|
kP (mg/g.min1/2): intraparticle diffusion rate constant |
|
|
| Arrhenius equation |
|
|
|
k0: Arrhenius constant
Ea (kJ /mol):activation energy |
|
|
| Clausius-Clapeyron equations |
|
|
|
∆Hx (kJ /mol): isosteric heat of adsorption |
| ∆H*(kJ /mol): activation enthalpy change |
| ∆S*(kJ/ mol.K): activation entropy change |
∆G*(kJ /mol): activation Gibbs energy change
kB: Boltzmann constant |
| Ke: equilibrium constant |
| ∆H°(kJ /mol): standard enthalpy change |
| ∆S°(kJ /mol.K): standard entropy change |
∆G°(kJ /mol): standard Gibbs free energy change
C0 (mg/ L): initial adsorbate concentration in solution |
| Error equations |
|
|
|
|
|
|
Table 4.
Parameters for Kinetic Models of Mo (VI) Sorption Onto Fe3O4/SiO2/P4VP
|
T(K)
|
q
e,exp
(mg/ g)
|
Pseudo First Order Kinetic Model
|
|
q
e,cal
(mg/ g)
|
k
1
(min
-1
)
|
|
R
2
|
∆q%
|
| 293 |
0.8425 |
0.9265 |
0.0279 |
|
0.9059 |
9.97 |
| 303 |
1.1187 |
0.4865 |
0.0423 |
|
0.8724 |
56.51 |
| 313 |
0.9575 |
0.7283 |
0.0265 |
|
0.9602 |
23.93 |
|
|
|
Pseudo Second Order Kinetic Model
|
|
|
|
q
e,cal
( mg/ g)
|
k
2
(g/ mg.min)
|
|
R
2
|
∆q%
|
| 293 |
0.8425 |
1.4883 |
0.0079 |
|
0.9146 |
76.65 |
| 303 |
1.1187 |
1.1608 |
0.1873 |
|
0.9969 |
3.76 |
| 313 |
0.9575 |
1.0352 |
0.0654 |
|
0.9848 |
8.11 |
|
|
|
Intraparticle Diffusion Model
|
|
|
|
q
e,cal
( mg/ g)
|
K
p
(g/ mg.min)
|
C
t
( mg/ g)
|
R
2
|
∆q%
|
| 293 |
0.8425 |
0.8454 |
0.07717 |
1.81×10-6 |
0.9516 |
0.34 |
| 303 |
1.1187 |
1.5095 |
0.1378 |
0.4293 |
0.1956 |
34.93 |
| 313 |
0.9575 |
1.1042 |
1.1042 |
0.1558 |
0.8571 |
15.32 |
Solution Volume = 25 mL; Initial Mo(VI) concentrations =2 mg/L; Sorbent Dosage=0.04g; pH=3.5.
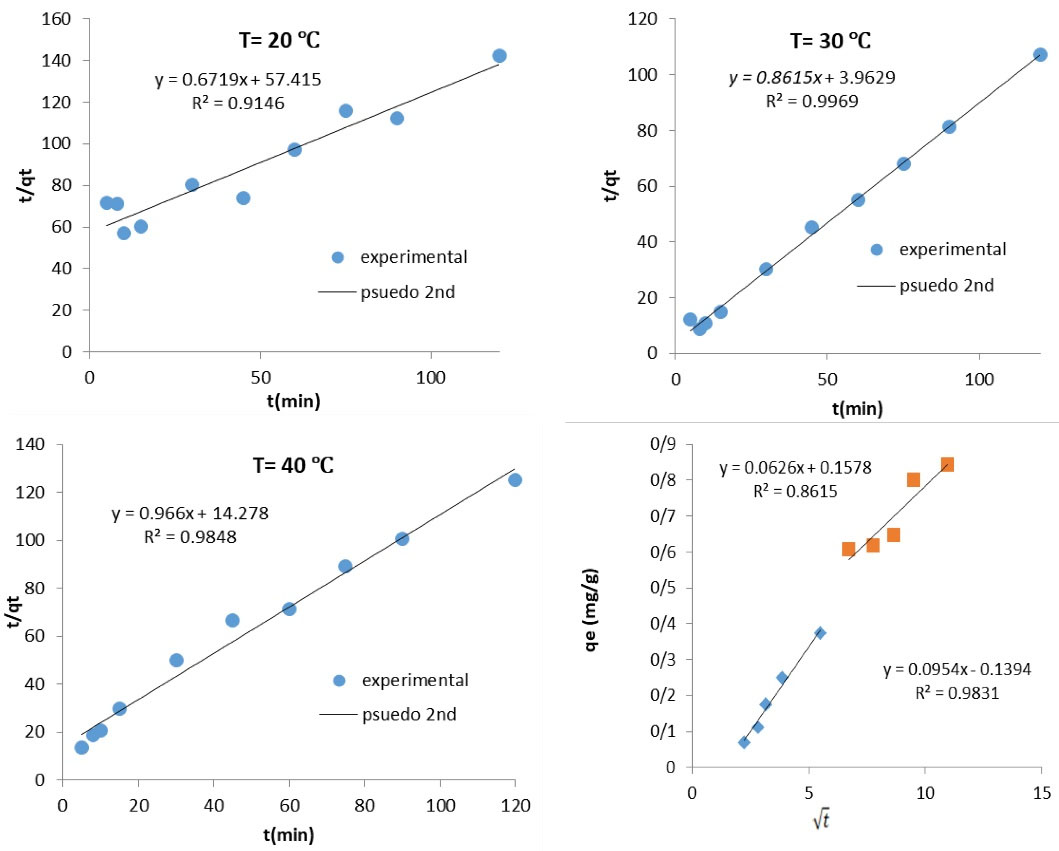
Figure 5.
Pseudo-Second-Order Plots and Webber Morris Model for the Mo(VI) Ions Removal Onto the Adsorbent.
.
Pseudo-Second-Order Plots and Webber Morris Model for the Mo(VI) Ions Removal Onto the Adsorbent.
3.2.7. Adsorption Isotherms and Thermodynamic Properties
Adsorption isotherm data obtained from the experiments were examined by the Generalized, Fritz Schlunder and Weber Van Vliet isotherm models. The forms can be explained by equations (see Table 3). The three models adsorption isotherms are presented in Table 5 and the curves are presented in Fig. 6.
Table 5.
Generalized, Fritz Schlunder, and Weber Van Vliet Isotherm Constants for the Adsorption of Mo(VI) Ions Onto Fe3O4/SiO2/P4VP
|
T(K)
|
Generalized Model
|
|
q
m
|
k
|
n
|
R
2
|
RMSE
|
χ
2
|
| 293 |
5.2856 |
3.1218 |
3.9821 |
0.9979 |
0.0777 |
0.0014 |
| 303 |
5.4652 |
3.7745 |
1.9390 |
0.9956 |
0.0944 |
0.0025 |
| 313 |
2.7503×1015 |
1.0461×1015 |
0.7766 |
0.9959 |
0.1046 |
0.0021 |
|
|
Fritz Schlunder Model
|
|
|
K
1
|
K
2
|
n
|
m
|
R
2
|
RMSE
|
χ
2
|
| 293 |
1.4012 |
0.1013 |
3.1126 |
4.0401 |
0.9988 |
0.0596 |
0.0005 |
| 303 |
1.1307 |
0.0152 |
1.4746 |
3.1363 |
0.9979 |
0.0646 |
0.0014 |
| 313 |
8.0934 |
2.0782 |
0.7766 |
1.8276×10-8 |
0.9959 |
0.1046 |
0.0021 |
|
|
Weber Van Vliet Model
|
|
|
k
|
n
|
m
|
l
|
R
2
|
RMSE
|
χ
2
|
| 293 |
0.9343 |
8.3131×10-5 |
5.1146 |
0.3498 |
0.9967 |
0.0370 |
0.0005 |
| 303 |
0.9291 |
1.1208×10-4 |
5.4726 |
0.7112 |
0.9989 |
0.0372 |
0.0004 |
| 313 |
0.3042 |
-18.4924 |
-0.0013 |
19.7058 |
0.9972 |
0.0446 |
0.0007 |
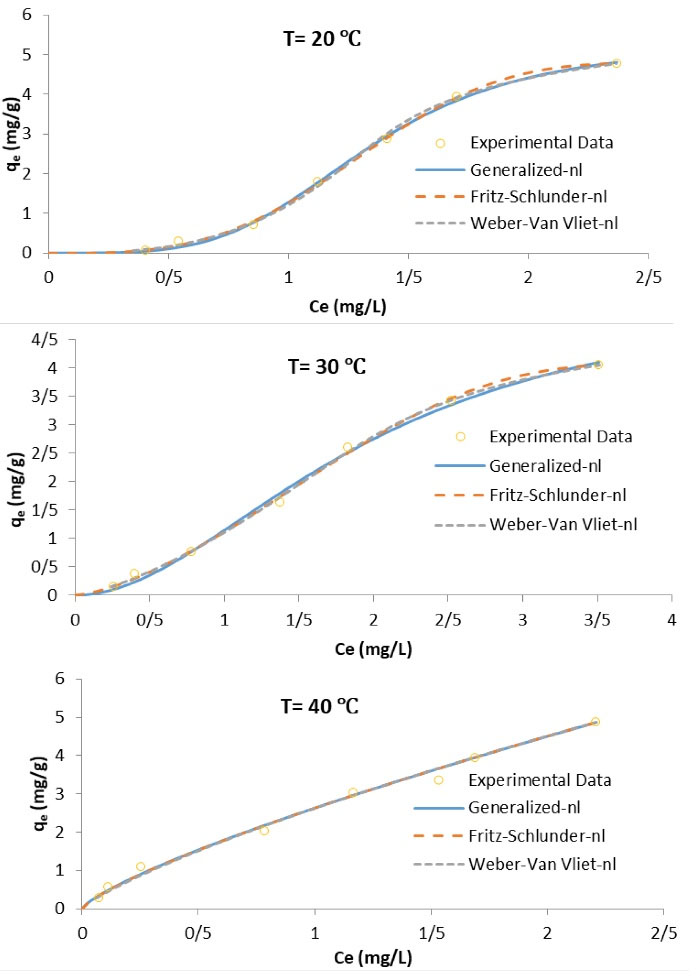
Figure 6.
Isotherm Curves Obtained by the Best Non-Linear Modeling at Different Temperatures.
.
Isotherm Curves Obtained by the Best Non-Linear Modeling at Different Temperatures.
The adsorption of Mo(IV) onto Fe3O4/SiO2/P4VP at different temperatures showed (Fig. 4F and Table 4) an ascent in the adsorption amount with rising temperatures. The standard Gibbs free energy change (ΔG°) is the basic principle of spontaneity. Reactions are spontaneous at a certain temperature when ΔG° has a negative value. The standard enthalpy change (ΔH°), standard entropy change (ΔS°) and standard Gibbs free energy change (ΔG°), which were associated with the adsorption course, were calculated using the equations (see Table 3). The plot of ln Kd vs. 1/T for the adsorption of Mo (VI) was drawn, which presented the values of ΔH° and ΔS° obtained from the slope and intercept, respectively. The ΔG° was also calculated based on ΔH° and ΔS° (see Table 3). The value of the obtained parameters was shown in Table 6. The ΔH° and ΔS° were found to be 61.30 kJ /mol and 209.84 J/mol K, respectively. The positive results of ΔH° suggested the endothermic nature of adsorption of the Mo(VI) ions on Fe3O4/SiO2/P4VP.
Table 6.
Thermodynamic Results of Mo(VI) Ions Adsorption by Fe3O4/SiO2/P4VP in Temperatures of 293, 303 and 313
|
Temperature (K)
|
ΔG
o
(kJ/ mol)
|
ΔH
o
( kJ/ mol)
|
ΔS
o
(J/ mol.K)
|
| 293 |
-0.177 |
61.30 |
209.84 |
| 303 |
-2.276 |
|
|
| 313 |
-0.374 |
|
|
The positive ΔS° results showed the growing possibility at the interface between solid and solution during the adsorption course. The positive ΔS° of adsorption also represented the affinity of the adsorbent for the Mo(VI) ion. Negative values of ΔG° for Mo(VI) adsorption by Fe3O4/SiO2/P4VP indicated that the adsorption process was spontaneous and feasible. Table 7 compares the results of this study with several similar works.
Table 7.
Comparison Between the Results of This Study and Some Similar Studies
|
Adsorbent
|
Optimal pH
|
Capacity
(mg/g)
|
Best Fitted Isotherm Model
|
Equilibrium Time (min)
|
Best Fitted Kinetic Model
|
Reference
|
| Fe3O4/SiO2/P4VP |
3.5 |
4.87 |
Weber Van Vliet |
210 |
Pseudo-second-order |
Present study |
| Modified resin with aniline formaldehyde |
5 |
3.1
4.03 |
Freundlich |
5 |
- |
(41) |
| Di-(2-ethylhexyl) phosphoric acid coated silanized magnetite nanoparticles |
- |
25.84 |
Langmuir |
- |
- |
(42) |
| Macroporous resin |
7.25 |
228.2 |
Freundlich |
25 |
- |
(43) |
| Nanometer-sized titanium dioxide |
1 |
2.01 |
|
|
|
(44) |
3.2.8. Effect of Foreign Ions
To judge the separation of the preconcentration system, the performance of several metal ions (5 mg/L) was investigated for the sorption behavior of Mo(VI) ions (5 mg/L). The results were displayed in Table 8. This table shows that the best competitive ions in adsorption of Mo(VI) ions onto Fe3O4/SiO2/P4VP, were Co(II) and Pt(IV). The significance of other foreign ions mentioned at certain concentrations was marginal. The adsorption of Mo(VI) ions on Fe3O4/SiO2/P4VP in the presence of all mentioned ions (solution volume=25 mL, adsorbent amount=0.04 g, concentration of each ion=5 mg/L) was evaluated. The Mo(VI) ions in the environmental samples can be made available quantitatively.
Table 8.
Effect of Interfering Ions on Sorption
|
Interfering Ion
|
A (mg/L)
|
%E
|
%L
|
D
|
| - |
4.75 |
95.04 |
0 |
59.40 |
| Cd 2+ |
4.47 |
89.40 |
5.89 |
55.88 |
| Ni2+ |
4.32 |
86.40 |
9.05 |
54.00 |
| Ca2+ |
4.43 |
88.52 |
6.74 |
55.32 |
| Co2+ |
4.14 |
84.50 |
12.84 |
52.81 |
| Fe 3+ |
4.46 |
89.40 |
6.10 |
55.88 |
| Ba2+ |
4.51 |
90.12 |
5.05 |
56.32 |
| Pt 4+ |
4.17 |
83.30 |
12.21 |
52.06 |
| Mixed Above |
4.15 |
83.00 |
12.63 |
51.88 |
A: Amount of adsorbed Mo(VI), L=(CeNo-ion-Ce)/CeNo-ion: Loss adsorption (%), E=(C0-Ce)/C0: Extraction percentage (%), and D=Q/Ce: distribution ratio
3.2.9. Application of Method
The Fe3O4/SiO2/P4VP was used to preconcentrate and determine Mo(VI) ions in two real samples (Tehran tap water and spring water). The pH of the water samples was adjusted to 3.5. After detecting Mo(VI) ions in the samples, the amounts of 0.2, 0.4 and 0.6 mg/L of Mo(VI) ions were added to 25 mL of these samples and mixed with 0.04 g of adsorbent and stirred for 3.5 hours. The obtained results are presented in Table 9. These results show the practical applicability for the Mo(VI) determination in samples.
Table 9.
Results Obtained for Mo(VI) Ion Determination in Real Samples
|
Water Samples
|
Found Mo(VI)
(Without Spiking) (mg/L)
|
Added
Mo(VI) (mg/L)
|
Found Mo(VI) Average
(After Spiking) (mg/L)
|
Pre-concentration Factor
|
Recovery (%)
|
SD
|
Relative SD (%)
a
|
| Tap water |
ND |
0.2
0.4
0.6 |
0.25
0.40
0.61 |
10
10
10 |
123.4
100.8
102.2 |
0.321
0.153
0.306 |
13.03
3.79
4.98 |
| Spring water |
0.12 |
0.2
0.4
0.6 |
0.27
0.42
0.72 |
10
10
10 |
83.3
80.1
99.5 |
0.153
0.153
0.208 |
5.73
3.67
2.90 |
ND: Not Detected; SD, standard deviation.
a, for three determinations
4. Conclusion
In this study, the adsorption isotherms, kinetic, and thermodynamic properties related to adsorption of Mo(VI) ions on Fe3O4/SiO2/P4VP were analyzed using batch-adsorption techniques. The magnetic P4VP was prepared by copolymerization of P4VP with TMSPMA. Several techniques such as FTIR, SEM, and XRD were used for the characterization of the prepared adsorbent. The optimum conditions were determined for Mo(VI) ions adsorption. The values of parameters in tests were: pH (3.5), adsorbent dose (0.04 g), initial Mo(VI) ions concentration (2.0 mg/L), contact time range (0 to 120 minutes), and temperatures (20, 30 and 40°C). In desorption studies, it was found that the 1M HCl was suitable for Mo(IV) ions recovery. The adsorption of Mo(VI) ions on Fe3O4/SiO2/P4VP tends to Kinetics of adsorption with high correlation Pseudo-kinetics of second order. The Ea value of 82.5497 kJ/mol reported in this study indicated that the adsorption had a higher potential in the context of chemisorption and because of the negative ΔG° and the positive ΔH°, the total adsorption course was endothermic and spontaneous. The equilibrium data were analyzed with Generalized, Fritz Schlunder and Weber Van Vliet isotherm models. The information gained from our investigation provided excellent results for the Fritz Schlunder model compared to other models. We determined the influence of other ions including Cd2+, Ca2+, Co2+, Fe3+, Ba2+, and Pt 4+ on the uptake of Mo(IV) ions. The results showed that the most effective ions in the uptake of Mo(IV) ions on Fe3O4/SiO2/P4VP were Co(II) and Pt(IV), while the effect of other foreign ions, which were inserted at certain concentrations, was found to be minor. The uptake of Mo(VI) ions on Fe3O4/SiO2/P4VP in the presence of all above-mentioned ions (solution volume=25 mL, adsorbent amount=0.0 4 g, concentration of each ion=5 mg/L) showed that the amount of Mo(VI) ions is measurable in the environmental samples.
Conflict of Interest Disclosures
The authors declare that they have no competing interests.
Acknowledgements
The authors would like to express their heartfelt thanks to all those who aided them with conducting this study, as well as to the nuclear science and technology research institute for financial and technical supports.
References
- El-Moselhy MM, Sengupta AK, Smith R. Carminic acid modified anion exchanger for the removal and preconcentration of Mo(VI) from wastewater. J Hazard Mater 2011; 185(1):442-6. doi: 10.1016/j.jhazmat.2010.09.052 [Crossref] [ Google Scholar]
- Arthur D. Interrelationships of molybdenum and copper in the diet of the guinea pig. J Nutr 1965; 87(1):69-76. doi: 10.1093/jn/87.1.69 [Crossref] [ Google Scholar]
- Shan W, Fang D, Zhao Z, Shuang Y, Ning L, Xing Z. Application of orange peel for adsorption separation of molybdenum(VI) from Re-containing industrial effluent. Biomass Bioenergy 2012; 37:289-97. doi: 10.1016/j.biombioe.2011.11.015 [Crossref] [ Google Scholar]
- Atia AA, Donia AM, Awed HA. Synthesis of magnetic chelating resins functionalized with tetraethylenepentamine for adsorption of molybdate anions from aqueous solutions. J Hazard Mater 2008; 155(1-2):100-8. doi: 10.1016/j.jhazmat.2007.11.035 [Crossref] [ Google Scholar]
- Pathak SK, Singh SK, Mahtele A, Tripathi SC. Studies on extraction behaviour of molybdenum(VI) from acidic radioactive waste using 2(ethylhexyl) phosphonic acids, mono 2(ethylhexyl) ester (PC-88A)/n-dodecane. J Radioanal Nucl Chem 2010; 284(3):597-603. doi: 10.1007/s10967-010-0511-y [Crossref] [ Google Scholar]
- Afkhami A, Conway BE. Investigation of removal of Cr(VI), Mo(VI), W(VI), V(IV), and V(V) oxy-ions from industrial waste-waters by adsorption and electrosorption at high-area carbon cloth. J Colloid Interface Sci 2002; 251(2):248-55. doi: 10.1006/jcis.2001.8157 [Crossref] [ Google Scholar]
- Afkhami A, Madrakian T, Amini A. Mo(VI) and W(VI) removal from water samples by acid-treated high area carbon cloth. Desalination 2009; 243(1):258-64. doi: 10.1016/j.desal.2008.04.028 [Crossref] [ Google Scholar]
- Wu CH, Lo SL, Lin CF. Competitive adsorption of molybdate, chromate, sulfate, selenate, and selenite on γ-Al2O3. Colloids Surf A Physicochem Eng Asp 2000; 166(1-3):251-9. doi: 10.1016/S0927-7757(99)00404-5 [Crossref] [ Google Scholar]
- Xu N, Christodoulatos C, Braida W. Adsorption of molybdate and tetrathiomolybdate onto pyrite and goethite: effect of pH and competitive anions. Chemosphere 2006; 62(10):1726-35. doi: 10.1016/j.chemosphere.2005.06.025 [Crossref] [ Google Scholar]
- Bostick BC, Fendorf S, Helz GR. Differential adsorption of molybdate and tetrathiomolybdate on pyrite (FeS2). Environ Sci Technol 2003; 37(2):285-91. doi: 10.1021/es0257467 [Crossref] [ Google Scholar]
- Faghihian H, Malekpour A, Maragheh MG. Adsorption of molybdate ion by natrolite and clinoptilolite-rich tuffs. Int J Environ Pollut 2002; 18(2):181-9. doi: 10.1504/ijep.2002.000704 [Crossref] [ Google Scholar]
- Ryden JC, Syers JK, Tillman RW. Inorganic anion sorption and interactions with phosphate sorption by hydrous ferric oxide gel. J Soil Sci 1987; 38(2):211-7. doi: 10.1111/j.1365-2389.1987.tb02138.x [Crossref] [ Google Scholar]
- Abd Ali ZT, Ismail ZZ. Experimental and modeling study of water defluoridation using waste granular brick in a continuous up-flow fixed bed. Environ Eng Res 2021; 26(2):190506. doi: 10.4491/eer.2019.506 [Crossref] [ Google Scholar]
- Griffiths DWL, Hallam HE, Thomas WJ. Infra-red study of adsorption and oxidation of ammonia on silica-supported platinum and silica. Trans Faraday Soc 1968; 64(0):3361-9. doi: 10.1039/tf9686403361 [Crossref] [ Google Scholar]
- Sun C, Lee JS, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev 2008; 60(11):1252-65. doi: 10.1016/j.addr.2008.03.018 [Crossref] [ Google Scholar]
- Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 2008; 108(6):2064-110. doi: 10.1021/cr068445e [Crossref] [ Google Scholar]
- Bucak S, Jones DA, Laibinis PE, Hatton TA. Protein separations using colloidal magnetic nanoparticles. Biotechnol Prog 2003; 19(2):477-84. doi: 10.1021/bp0200853 [Crossref] [ Google Scholar]
- White BR, Stackhouse BT, Holcombe JA. Magnetic gamma-Fe2O3 nanoparticles coated with poly-l-cysteine for chelation of As(III), Cu(II), Cd(II), Ni(II), Pb(II) and Zn(II). J Hazard Mater 2009; 161(2-3):848-53. doi: 10.1016/j.jhazmat.2008.04.105 [Crossref] [ Google Scholar]
- Savić AB, Čokeša D, Savić Biserčić M, Častvan-Janković I, Petrović R, Živković LS. Multifunctional use of magnetite-coated tuff grains in water treatment: removal of arsenates and phosphates. Adv Powder Technol 2019; 30(8):1687-95. doi: 10.1016/j.apt.2019.05.020 [Crossref] [ Google Scholar]
- Wang Y, Sun D, Liu G, Jiang W. Synthesis of Fe3O4@SiO2@ZnO core–shell structured microspheres and microwave absorption properties. Adv Powder Technol 2015; 26(6):1537-43. doi: 10.1016/j.apt.2015.08.014 [Crossref] [ Google Scholar]
- Hosseini M, Esrafili A, Yegane badi M, Gholami M. New magnetic/Biosilica/Sodium Alginate Composites for removal of Pb(II) ions from aqueous solutions: kinetic and isotherm studies. J Adv Environ Health Res 2018; 6(3):160-72. doi: 10.22102/jaehr.2018.126798.1073 [Crossref] [ Google Scholar]
- Zhou L, Wang Y, Liu Z, Huang Q. Characteristics of equilibrium, kinetics studies for adsorption of Hg(II), Cu(II), and Ni(II) ions by thiourea-modified magnetic chitosan microspheres. J Hazard Mater 2009; 161(2-3):995-1002. doi: 10.1016/j.jhazmat.2008.04.078 [Crossref] [ Google Scholar]
- Soto D, Urdaneta J, Pernía K, León O, Muñoz-Bonilla A, Fernández-García M. Heavy metal (Cd2+, Ni2+, Pb2+ and Ni2+) adsorption in aqueous solutions by oxidized starches. Polym Adv Technol 2015; 26(2):147-52. doi: 10.1002/pat.3439 [Crossref] [ Google Scholar]
- Hai B, Wu J, Chen X, Protasiewicz JD, Scherson DA. Metal-ion adsorption on carboxyl-bearing self-assembled monolayers covalently bound to magnetic nanoparticles. Langmuir 2005; 21(7):3104-5. doi: 10.1021/la0487139 [Crossref] [ Google Scholar]
- Ngomsik AF, Bee A, Draye M, Cote G, Cabuil V. Magnetic nano- and microparticles for metal removal and environmental applications: a review. C R Chim 2005; 8(6):963-70. doi: 10.1016/j.crci.2005.01.001 [Crossref] [ Google Scholar]
- Vilela PB, Dalalibera A, Duminelli EC, Becegato VA, Paulino AT. Adsorption and removal of chromium (VI) contained in aqueous solutions using a chitosan-based hydrogel. Environ Sci Pollut Res Int 2019; 26(28):28481-9. doi: 10.1007/s11356-018-3208-3 [Crossref] [ Google Scholar]
- Khosravi M, Azizian S. Synthesis of different nanostructured flower-like iron oxides and study of their performance as adsorbent. Adv Powder Technol 2014; 25(5):1578-84. doi: 10.1016/j.apt.2014.05.010 [Crossref] [ Google Scholar]
- Zhu J, Wei S, Chen M, Gu H, Rapole SB, Pallavkar S. Magnetic nanocomposites for environmental remediation. Adv Powder Technol 2013; 24(2):459-67. doi: 10.1016/j.apt.2012.10.012 [Crossref] [ Google Scholar]
- Zou C, Jiang W, Liang J, Sun X, Guan Y. Removal of Pb(II) from aqueous solutions by adsorption on magnetic bentonite. Environ Sci Pollut Res Int 2019; 26(2):1315-22. doi: 10.1007/s11356-018-3652-0 [Crossref] [ Google Scholar]
- De Rossi A, Rigon MR, Zaparoli M, Braido RD, Colla LM, Dotto GL. Chromium (VI) biosorption by Saccharomyces cerevisiae subjected to chemical and thermal treatments. Environ Sci Pollut Res Int 2018; 25(19):19179-86. doi: 10.1007/s11356-018-2377-4 [Crossref] [ Google Scholar]
- Yetimoğlu EK, Fırlak M, Kahraman MV, Deniz S. Removal of Pb2+ and Cd2+ ions from aqueous solutions using guanidine modified hydrogels. Polym Adv Technol 2011; 22(5):612-9. doi: 10.1002/pat.1554 [Crossref] [ Google Scholar]
- Maddodi SA, Alalwan HA, Alminshid AH, Abbas MN. Isotherm and computational fluid dynamics analysis of nickel ion adsorption from aqueous solution using activated carbon. S Afr J Chem Eng 2020; 32:5-12. doi: 10.1016/j.sajce.2020.01.002 [Crossref] [ Google Scholar]
- Krueyai Y, Punyapalakul P, Wongrueng A. Removal of haloacetonitrile by adsorption on thiol-functionalized mesoporous composites based on natural rubber and hexagonal mesoporous silica. Environ Eng Res 2015; 20(4):342-6. doi: 10.4491/eer.2015.070 [Crossref] [ Google Scholar]
- Tanev PT, Pinnavaia TJ. Mesoporous silica molecular sieves prepared by ionic and neutral surfactant templating: a comparison of physical properties. Chem Mater 1996; 8(8):2068-79. doi: 10.1021/cm950549a [Crossref] [ Google Scholar]
- Altun T, Ecevit H. Cr(VI) removal using Fe2O3-chitosan-cherry kernel shell pyrolytic charcoal composite beads. Environ Eng Res 2020; 25(3):426-38. doi: 10.4491/eer.2019.112 [Crossref] [ Google Scholar]
- Baile P, Vidal L, Canals A. A modified zeolite/iron oxide composite as a sorbent for magnetic dispersive solid-phase extraction for the preconcentration of nonsteroidal anti-inflammatory drugs in water and urine samples. J Chromatogr A 2019; 1603:33-43. doi: 10.1016/j.chroma.2019.06.039 [Crossref] [ Google Scholar]
- Ulusoy Hİ, Yılmaz E, Soylak M. Magnetic solid phase extraction of trace paracetamol and caffeine in synthetic urine and wastewater samples by a using core shell hybrid material consisting of graphene oxide/multiwalled carbon nanotube/Fe3O4/SiO2. Microchem J 2019; 145:843-51. doi: 10.1016/j.microc.2018.11.056 [Crossref] [ Google Scholar]
- Zhai Y, He Q, Han Q, Duan S. Solid-phase extraction of trace metal ions with magnetic nanoparticles modified with 2,6-diaminopyridine. Microchim Acta 2012; 178(3-4):405-12. doi: 10.1007/s00604-012-0857-7 [Crossref] [ Google Scholar]
- Chakraborty S, Chowdhury S, Das Saha P. Adsorption of crystal violet from aqueous solution onto NaOH-modified rice husk. Carbohydr Polym 2011; 86(4):1533-41. doi: 10.1016/j.carbpol.2011.06.058 [Crossref] [ Google Scholar]
- Walcarius A, Delacôte C. Rate of access to the binding sites in organically modified silicates 3 Effect of structure and density of functional groups in mesoporous solids obtained by the co-condensation route. Chem Mater 2003; 15(22):4181-92. doi: 10.1021/cm031089l [Crossref] [ Google Scholar]
- Borowiak-Resterna A, Cierpiszewski R, Prochaska K. Kinetic and equilibrium studies of the removal of cadmium ions from acidic chloride solutions by hydrophobic pyridinecarboxamide extractants. J Hazard Mater 2010; 179(1-3):828-33. doi: 10.1016/j.jhazmat.2010.03.078 [Crossref] [ Google Scholar]
- Mallakpour S, Barati A. Efficient preparation of hybrid nanocomposite coatings based on poly(vinyl alcohol) and silane coupling agent modified TiO2 nanoparticles. Prog Org Coat 2011; 71(4):391-8. doi: 10.1016/j.porgcoat.2011.04.010 [Crossref] [ Google Scholar]
- Sid Kalal H, Ahmad Panahi H, Framarzi N, Moniri E, Naeemy A, Hoveidi H. New chelating resin for preconcentration and determination of molybdenum by inductive couple plasma atomic emission spectroscopy. Int J Environ Sci Technol 2011; 8(3):501-12. doi: 10.1007/bf03326236 [Crossref] [ Google Scholar]
- Agarwal S, Tyagi I, Gupta VK, Hanifpour F, Maghsudi M, Javadian H. Mo (IV) adsorption from nitric acid media by Di-(2-ethylhexyl) phosphoric acid (D2EHPA) coated silanized magnetite nanoparticles. J Mol Liq 2016; 218:346-53. doi: 10.1016/j.molliq.2016.02.057 [Crossref] [ Google Scholar]