Avicenna J Environ Health Eng. 7(2):66-71.
doi: 10.34172/ajehe.2020.10
Original Article
Taguchi Optimization of Catalytic Ozonation Process Using Modified Bone Char Ash for Removal of Methylene Blue from Aqueous Solution
Ghorban Asgari 1, 2  , Somaye Akbari 3, *
, Somaye Akbari 3, * 
Author information:
1Social Determinants of Health Research Center (SDHRC), Hamadan University of Medical Sciences Hamadan, Iran
2Department of Environmental Health Engineering, School of Public Health, Hamadan University of Medical Sciences, Hamadan, Iran
3Department of Environmental Health Engineering, Faculty of Medical Sciences, Lecturer of Ghand-e- Karaj International Applied and Technology Center, Alborz, Iran
Abstract
Methylene blue (MB) dye is an environmental contaminant that has been mostly used in textile industry. Taguchi orthogonal array design was employed as an optimization method to reduce the number of experiments. In this research, bone char ash modified by MgO-Fe catalyst was applied for degradation of MB in catalytic ozonation process (COP) system and operational parameters including initial MB dosages, initial pH, catalyst dose, and contact time were optimized with Taguchi method. Accordingly, the best condition for the removal of MB obtained at initial MB concentration of 20 mg/L, reaction time of 15 minutes, initial pH value of 10, and catalyst concentration of 0.1 g/L. Additionally, optimization of experimental set-up showed that the MB concentration had a notable effect on MB degradation in COP process (55.6%), and reaction time had a negligible effect (1.98%). At this condition, total organic carbon (TOC) removal was determined to be 31% but in longer time, its removal increased to 65%.
Keywords: Methylene blue, Bone char ash, MgO, Taguchi
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
In recent decades, dyes have been extensively applied in textile industries. These industries generate high amounts of colored effluents that are discharged into the environment without any treatment (1,2). These dyes have adverse effects on the environment due to their carcinogenicity, mutagenicity, toxicity, allergenicity, and aesthetic effects (3,4). Methylene blue (MB) dye is most widely consumed for dying because of its favorable characteristics such as transparency, ease of use, and low energy consumption. MB has adverse impacts on human and animal health; for example, contact with MB can disturb eye function. Additionally, it has acute respiratory and gastrointestinal effects, such as decreased lung respiratory capacity, vomiting, and methemoglobinemia. According to previous studies, the recommended limit for MB in the surface water is 0.2 mg/L (5,6).
Therefore, it must be removed before being released into the environment to protect the environment and human health (7). Conventional methods are used for textile effluent treatment such as biological, chemical, adsorption, and electrochemical processes (8). It has been reported that the biological procedure cannot be applied as the main method for the treatment of textile effluents. Adsorption is one of the most effective methods for removing dye, which has some disadvantages such as high cost and remaining of the adsorbent material in the treated solution (9). Advanced oxidation processes (AOPs) are evaluated as appropriate procedures for degradation of refractory materials. Ozonation is one of the AOPs which is commonly used for treating industrial effluents. In recent years, ozonation has been widely used for dye removal because of its high oxidizing properties. However, many studies have reported that the application of single ozone has low efficiency for the degradation of refractory and non-degradable compounds (10-12). As mentioned above, single ozonation cannot oxidize contaminants completely and may produce toxic and dangerous intermediate compounds. Therefore, catalytic ozonation process (COP) has been developed for enhancing the yield of ozonation (13-15). Nowadays, several materials such as metal, metal oxide, metal ions, metal oxides, modified minerals, and adsorbent substances have been studied as a catalyst. The addition of the catalyst can lead to an increase in ozone decomposition and generate more MB radicals. The increase in MB radicals will be beneficial for the degradation of refractory compounds (16). According to the kind of catalyst, COP can be divided into two main types: heterogeneous and homogeneous. Heterogeneous processes, due to easier separation of the catalyst at the end of the experiment, have higher efficiency for pollutant degradation (13,17). Finding a low-cost catalyst is a challenge in contaminant removal. Due to the human need for food, especially meat, billions of animals are slaughtered annually for food in the world, which can lead to the production of bone waste. Therefore, bone char can be produced from bone waste and used as a suitable catalyst in COP due to low cost, accessibility, and high physical strength (18). Several studies have reported the modification of bone char (19,20). Nanoparticles such as magnesium oxide (MgO) can increase the efficiency of bone char in the removal of MB dye. MgO is a variety of metal oxides that have been broadly applied for elimination of many contaminants. It is categorized as a favorable component because of low cost, non-toxicity, steadiness, and high activity. Furthermore, reactivity and surface properties of MgO can be improved by doping with metal and nonmetal elements (16,21). By increasing the number of parameters and their levels, the use of statistical method is necessary to optimize the process conditions. Taguchi orthogonal array design L16 is a statistical method that can be employed for process optimization. This method can significantly reduce the experimental cost and time required (22,23).
In this experiment, a catalyst with high activity for degradation of MB is presented. Accordingly, the simultaneous doping of Fe and MgO on bone char is reported as the most important innovation in the present study. To the best of our knowledge, this is the first study on the modification of bone char using MgO and Fe nanoparticles and its application for dye wastewater treatment by COP process and Taguchi method.
2. Material and Methods
2.1. Chemicals and Catalyst Preparation
MB was obtained from Alvan dye Company, and magnesium chloride, iron nitrate, acid sulfuric, and hydroxide potassium were provided by Merck Chemicals. bone char ash was made in laboratory condition at 800ºC with electric furnaces for 2 hours. Then, bone char ash was powdered by an electric mill and graded using ASTM (mesh size 8-16). Bone char ash was modified by MgCl2. Afterwards, 4 g of bone char powder was mixed with MgCl2 (1M) and hydroxide potassium (1N) at 120 rpm. The prepared solution was dried at 70ºC in an oven for 22 hours and then the sample was cooled in a desiccator and calcinated in electric furnaces at 450ºC for 3 hours. In order to synthesize bone char/MgO/Fe, 1 g of iron nitrate (0.1 M) was added to the above-mentioned solution and all procedures were repeated for preparation of the catalyst (24).
2.2. Experimental Set-up
All tests were carried out under lab conditions and in a glass reactor. Ozone was produced from the pure oxygen in the ozone generator (COG-40A, ARDA, France) with a volume of 5 g/h (Fig. 1). The capacity of ozone gas in the input and output of the set up was calculated by the KI and thiosulfate methods, and ozone consumption in the impinger was measured by the ozone gas difference in input and output based on mg/L (7).
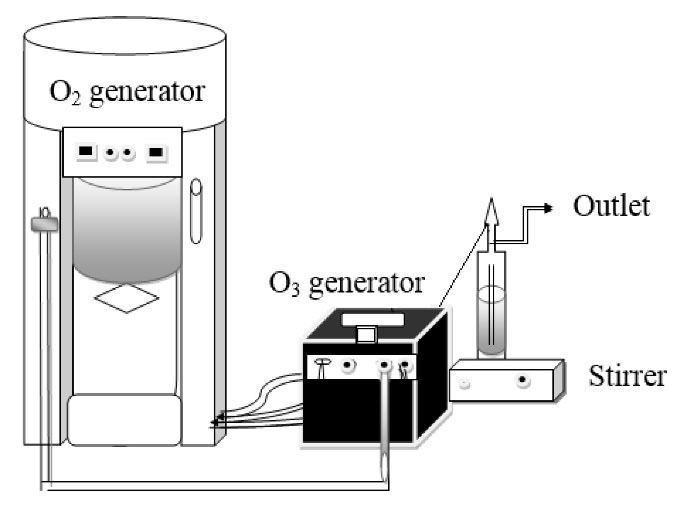
Figure 1.
Schematic Image of Ozonation Set-up
.
Schematic Image of Ozonation Set-up
2.3. Analytical Methods
The MB concentration was reported using UV-visible spectrophotometer (DR5000) at 663 nm wavelength. The pHZPC of as-prepared catalyst was measured by sodium NaCl (0.01 M) as an electrolyte. In addition, HCL (0.1N) and sodium hydroxide (0.1N) were used to adjust the solution pH. MB mineralization was determined based on COD removal (25).
2.4. Determination and Optimization of MB Degradation Experiment
Minitab Statistical Software (version 16) was applied to design the experimental set-up. In this study, the effect of main factors such as MB concentration, catalyst dosage, pH, and contact time on MB degradation were optimized by Taguchi method. Each parameter was determined at 4 levels (Table 1). All tests were performed in duplicate and the mean values have been used for calculations.
Table 1.
Controllable Factors and Their Rate
|
Designation
|
Explanation
|
Rate 1
|
Rate 2
|
Rate 3
|
Rate 4
|
| D1 |
Reaction time (min) |
5 |
10 |
15 |
20 |
| D2 |
Initial MB Concentration (mg/L) |
20 |
50 |
100 |
200 |
| D3 |
Solution pH |
2 |
4 |
8 |
10 |
| D4 |
Catalyst dose (g/L) |
0.1 |
0.3 |
0.5 |
0.7 |
The signal-to-noise (S/N) ratio was suggested to determine the output. Among 3 resulted ranges of S/N ratio, the highest one was considered as the optimum rate (equation 1) (22).
(1)
Maximum removal efficiency (MRE) (%) for MB degradation was calculated as follows (equation 2) (23).
where C0 and Ce are initial and final concentrations of MB, respectively.
In order to obtain the optimum condition for the MB degradation, the relationship between each factor and rate of contribution (%) in COP, the analysis of mean (ANOM) and variance (ANOVA) were applied.
Primarily, the mean of the S/N ratio (MS/N) of each parameter at a certain range was calculated (equation 3) (23).
(3)
Afterwards, the highest MS/N value was selected as the optimum condition of each factor as assessed in Taguchi method. The influence rate of each factor in MB degradation through COP was obtained via substituting the factorial sum of squares (SSF) (equation 4), the total sum of square (SST) (equation 5) and the variance of error (VE) (equation 6) in (equation 7):
(4)
(5)
(7)
3. Results and Discussion
3.1. Catalyst Characterization
The evolution of surface morphology of as-prepared catalyst was analyzed by field emission scanning electron microscopy (Fig. 2). Accordingly, the surface of the catalyst was porous and heterogeneous. Moreover, the available surface of the catalyst using BELSORP software was calculated to be 14 mg/L.

Figure 2.
SEM Pattern of Prepared Catalyst.
.
SEM Pattern of Prepared Catalyst.
3.2. Optimum Conditions
According to Taguchi method (L16), 4 factors including initial MB rate, catalyst dosage, pH, and contact time at 4 levels were selected for removal of MB by COP. The yield of each run was attained according to equations 1 & equations 2 and results were shown in Table 2.
Table 2.
The S/N Ratio of Each Experiment from Different Arrangement of Factors
|
Step
|
D
1
|
D
2
|
D
3
|
D
4
|
MRE, %
|
MRE, %
|
S/N
|
| 1 |
5 |
10 |
2 |
0.1 |
92 |
94 |
37.10 |
| 2 |
5 |
50 |
4 |
0.3 |
93 |
81 |
37.01 |
| 3 |
5 |
100 |
8 |
0.5 |
78 |
77 |
39.90 |
| 4 |
5 |
200 |
10 |
0.7 |
81 |
60 |
38.01 |
| 5 |
10 |
10 |
10 |
0.3 |
91 |
94 |
39.08 |
| 6 |
10 |
50 |
8 |
0.1 |
95 |
90 |
37.90 |
| 7 |
10 |
100 |
4 |
0.7 |
91 |
86 |
38.10 |
| 8 |
10 |
200 |
2 |
0.5 |
63 |
60 |
36.10 |
| 9 |
15 |
10 |
4 |
0.5 |
92 |
93 |
39.20 |
| 10 |
15 |
50 |
2 |
0.7 |
95 |
94 |
38.20 |
| 11 |
15 |
100 |
10 |
0.1 |
76 |
84 |
37.02 |
| 12 |
15 |
200 |
8 |
0.3 |
88 |
89 |
37.9 |
| 13 |
20 |
10 |
8 |
0.7 |
92 |
93 |
37.99 |
| 14 |
20 |
50 |
10 |
0.5 |
98 |
99 |
36.90 |
| 15 |
20 |
100 |
2 |
0.3 |
87 |
93 |
37.99 |
| 16 |
20 |
200 |
4 |
0.1 |
81 |
89 |
38.99
|
The S/N rate of each test from various arrangements of parameters with the boldface referring to the maximum value of S/N ratio among the 16 runs was indicated in Table 2. Taguchi described the S/N ratio in 3 ways: 1) the lower is better, 2) the highest is better, and 3) typically the S/N ratio should be high. As seen in Table 3, the optimum condition for MB degradation is as follow: initial MB concentration of 20 mg/L, catalyst dose of 0.1 g/L, pH value of 10, and reaction time of 15 minutes. Table 1 represents the coded values of the parameters.
Table 3.
Measurement of MS/N Ratios
|
Rate
|
Designation
|
|
D
1
|
D
2
|
D
3
|
D
4
|
| 1 |
37.10 |
39.90 |
37.99 |
38.99 |
| 2 |
39.08 |
37.90 |
38.10 |
36.40 |
| 3 |
39.20 |
38.20 |
37.01 |
37.99 |
| 4 |
37.99 |
37.01 |
38.20 |
37.90 |
3.3. Reaction Time
In this stage, four levels of reaction time (5, 10, 15, and 20 minutes) were examined to determine the effect of reaction time. Fig. 3 shows the effect of reaction time on MB degradation in COP process.
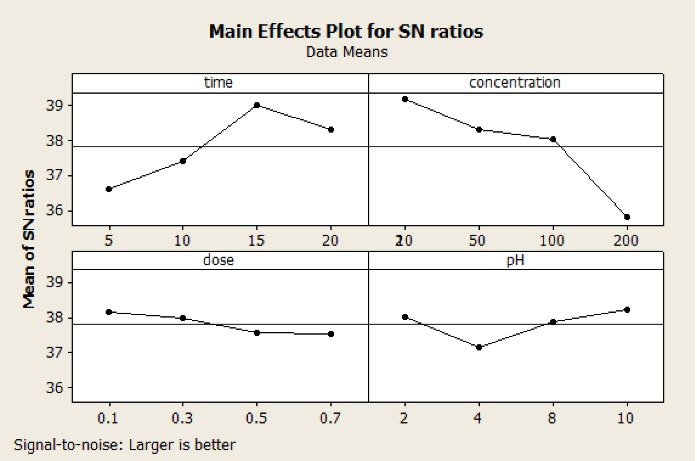
Figure 3.
The Effects of Operational Parameters on S/N Ratio for MB Removal.
.
The Effects of Operational Parameters on S/N Ratio for MB Removal.
As expected, the increase in reaction time enhanced COP degradation efficiency. The best result of dye removal was shown in 15 minutes with the S/N ratio of 39.20. In the longer reaction time, the amount of hydroxyl radicals increased based on the decomposition of the ozone. Therefore, the reaction between the OH and MB was facilitated and the yield increased. These findings are consistent with other studies (16).
3.4. Initial MB Concentration
The MB concentration for COP system was studied at 20, 50, 100, 200 mg/L. As indicated in Table 3, the S/N rate for MB dosages decreased from 39.90 to 37.01 with enhancing its level from 20 to 200 mg/L, respectively.
The initial concentration of MB is an important parameter in COP system. According to the results shown in Fig. 3, the higher removal rate was obtained at a lower initial MB level. It can be stated that at high concentration, the molecule of dye increased but the production of OH decreased. Additionally, the formation of intermediates at high initial MB concentration can consume ozone (26). Sui et al reported the same finding in the degradation of ciprofloxacin in COP process (27).
3.5. Solution pH
Fig. 3 depicts the effect of pH (2, 4, 8, and 10) on COP process. Based on the results, the maximum MB efficiency was achieved at pH of 10 with S/N ratio of 38.20. As it is known, pH has an effective role in ozone degradation in both direct oxidation (O3) and indirect oxidation (OH radical). Removal of MB was obtained in alkaline condition (pH = 10). In this pH, ozone decomposition increased and production of OH and HO2∙ accelerated. On the other hand, decomposition reactions in surface sites on the bone char-MgO-Fe with ozone caused production of many active radicals (28).
In this study, the pHzpc of bone char- MgO- Fe was obtained in a range of 8.95 and pHzpc was lower than solution pH. Accordingly, the removal percentage of MB decreased in acidic condition and increased in alkaline condition. Characteristics of catalyst surface were related to pHzpc. At pH>pHzpc, the catalyst surface was charged negatively and the interaction of ozone with strong Lewis acid on metal oxides surface sites increases removal efficiency (28,29). Moussavi et al reported higher phenol removal rates by ozonation under alkaline condition (pH = 8). They attributed this result to pHzpc because at higher alkaline conditions, the number of negative charges on catalyst surface increased so the removal of phenol decreased. Moreover, decomposition reactions at high pH values are elevated by the presence of hydroxide ions and removal efficiency is much better compared to low pH (30). A similar result was gained for MB removal at pH = 10 by Sousa et al (31).
3.6. Catalyst Dosage
The influence of catalyst dose on MB degradation was checked at doses of 0.1, 0.3, 0.5, 0.7 g/L. As observed in Fig. 3, when catalyst dose was 0.1g/L, the S/N ratio was 38.99. Lower S/N ratio was obtained when the catalyst dose exceeded 0.1 mg/L. Therefore, catalyst dose of 0.1 g/L was selected as the optimum condition. The effect of catalyst concentration can be attributed to the expansion of surface area of the catalyst and the availability of more active sites for ozone decomposition.
Furthermore, the presence of CaO and P2O5 in bone char structure in the form of (Ca5 (PO4)3OH) affected the generation of radical species (32). A further increase in catalyst dose (up to 0.1 g/L) did not significantly affect the percentage of dye removal. It is described by the kind of catalyst, the reaction condition, and the type of contaminant. Therefore, at lower doses, the surface active site of the catalyst was inadequate and was filled rapidly with MB molecules so by increasing the catalyst dosage, the contact rate between the pollutant and as-prepared catalyst increased. These results are in agreement with those of a study by Kruanak et al (15).
3.7. Percentage of Contribution
The contribution of each factor is the key to controlling the dye removal by COP system. Accordingly, the initial MB concentration is the most significant parameter with an approximate contribution of 55.6% in MB removal. The catalyst dosage was the second important factor with 16.32% contribution, followed by pH with 5.95% contribution. The least influential factor was the reaction time with 1.98% contribution. Therefore, the COP yield in MB degradation depends on the MB concentration and catalyst dosage.
3.8. Mineralization and MB Degradation
Degradation and mineralization of MB were determined in the optimum condition (pH = 10, catalyst dose = 0.1 g/L, reaction time = 15 min, and initial MB concentration = 20 mg/L). The reduction in COD value in COP process results in the mineralization progress of dye molecules and color removal. As indicated in Fig. 4, maximum removal (96%) of the MB was obtained at 15 minutes while COD removal efficiency was 31% in the same time. Moreover, the mineralization rate increased from 31% in 15 minutes to 65% in 30 minutes.
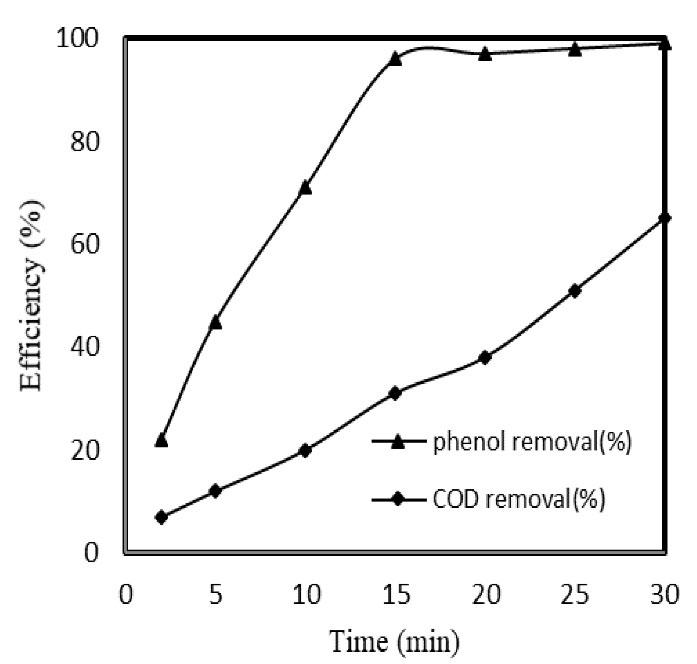
Figure 4.
MB and COD Removal in Optimum Condition (Initial MB Concentration: 20 mg/L, as-prepared catalyst rate: 0.1 g/L, and pH: 10)
.
MB and COD Removal in Optimum Condition (Initial MB Concentration: 20 mg/L, as-prepared catalyst rate: 0.1 g/L, and pH: 10)
It can be deduced that the resistant material to degradation is produced during the reaction in COP process. Furthermore, these molecules have a lower reactivity toward hydroxyl radicals and could decrease COD removal efficiency rate (32), which is reported by Subramani et al for indigo carmine dye removal (33).
4. Conclusion
Bone char ash was modified with MgO-Fe in COP process for the removal of MB. Optimum conditions for degradation of MB dye were found at catalyst dosage of 0.1 g/L, reaction time of 15 minutes, initial phenol concentration of 20 mg/L, and pH of 10. Moreover, the application of Taguchi method for the optimization of experimental set-up revealed that the initial MB concentration had the highest effect on degradation efficiency in COP process (55.6%), and reaction time had the lowest contribution (1.98%). According to SEM analysis, surface of the catalyst was porous and heterogeneous. Moreover, the available surface of the catalyst was calculated to be 14 mg/L. The as-prepared catalyst showed strong activity for MB removal. Additionally, these results suggested that optimization of COP process by Taguchi method could be effective in terms of cost and yield. Hence, bone char-MgO-Fe can be used as a sustainable and effective catalyst for the removal of organic and inorganic contaminants.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
Acknowledgments
This study was performed at Hamadan University of Medical Sciences.
References
- Muniyasamy A, Sivaporul G, Gopinath A, Lakshmanan R, Altaee A, Achary A. Process development for the degradation of textile azo dyes (mono-, di-, poly-) by advanced oxidation process-ozonation: experimental & partial derivative modelling approach. J Environ Manage 2020; 265:110397. doi: 10.1016/j.jenvman.2020.110397 [Crossref] [ Google Scholar]
- Hien NT, Nguyen LH, Van HT, Nguyen TD, Nguyen THV, Chu THH. Heterogeneous catalyst ozonation of Direct Black 22 from aqueous solution in the presence of metal slags originating from industrial solid wastes. Sep Purif Technol 2020; 233:115961. doi: 10.1016/j.seppur.2019.115961 [Crossref] [ Google Scholar]
- Ballav N, Debnath S, Pillay K, Maity A. Efficient removal of Reactive Black from aqueous solution using polyaniline coated ligno-cellulose composite as a potential adsorbent. J Mol Liq 2015; 209:387-96. doi: 10.1016/j.molliq.2015.05.051 [Crossref] [ Google Scholar]
- Hao YF, Yan LG, Yu HQ, Yang K, Yu SJ, Shan RR. Comparative study on adsorption of basic and acid dyes by hydroxy-aluminum pillared bentonite. J Mol Liq 2014; 199:202-7. doi: 10.1016/j.molliq.2014.09.005 [Crossref] [ Google Scholar]
- Rafatullah M, Sulaiman O, Hashim R, Ahmad A. Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 2010; 177(1-3):70-80. doi: 10.1016/j.jhazmat.2009.12.047 [Crossref] [ Google Scholar]
- Chanu LA, Singh WJ, Singh KJ, Devi KN. Effect of operational parameters on the photocatalytic degradation of Methylene blue dye solution using manganese doped ZnO nanoparticles. Results Phys 2019; 12:1230-7. doi: 10.1016/j.rinp.2018.12.089 [Crossref] [ Google Scholar]
- Hu E, Shang S, Chiu AK. Removal of reactive dyes in textile effluents by catalytic ozonation pursuing on-site effluent recycling. Molecules 2019; 24(15). doi: 10.3390/molecules24152755 [Crossref]
- Hu E, Wu X, Shang S, Tao XM, Jiang SX, Gan L. Catalytic ozonation of simulated textile dyeing wastewater using mesoporous carbon aerogel supported copper oxide catalyst. J Clean Prod 2016; 112:4710-8. doi: 10.1016/j.jclepro.2015.06.127 [Crossref] [ Google Scholar]
- Wu CH, Kuo CY, Chang CL. Decolorization of CI Reactive Red 2 by catalytic ozonation processes. J Hazard Mater 2008; 153(3):1052-8. doi: 10.1016/j.jhazmat.2007.09.058 [Crossref] [ Google Scholar]
- Huang Y, Luo M, Xu Z, Zhang D, Li L. Catalytic ozonation of organic contaminants in petrochemical wastewater with iron-nickel foam as catalyst. Sep Purif Technol 2019; 211:269-78. doi: 10.1016/j.seppur.2018.09.080 [Crossref] [ Google Scholar]
- Rosenfeldt EJ, Linden KG, Canonica S, von Gunten U. Comparison of the efficiency of *OH radical formation during ozonation and the advanced oxidation processes O3/H2O2 and UV/H2O2. Water Res 2006; 40(20):3695-704. doi: 10.1016/j.watres.2006.09.008 [Crossref] [ Google Scholar]
- Gonçalves AG, Órfão JJ, Pereira MF. Catalytic ozonation of sulphamethoxazole in the presence of carbon materials: catalytic performance and reaction pathways. J Hazard Mater 2012; 239-240:167-74. doi: 10.1016/j.jhazmat.2012.08.057 [Crossref] [ Google Scholar]
- Moussavi G, Khosravi R. Preparation and characterization of a biochar from pistachio hull biomass and its catalytic potential for ozonation of water recalcitrant contaminants. Bioresour Technol 2012; 119:66-71. doi: 10.1016/j.biortech.2012.05.101 [Crossref] [ Google Scholar]
- Li X, Chen W, Ma L, Huang Y, Wang H. Characteristics and mechanisms of catalytic ozonation with Fe-shaving-based catalyst in industrial wastewater advanced treatment. J Clean Prod 2019; 222:174-81. doi: 10.1016/j.jclepro.2019.03.084 [Crossref] [ Google Scholar]
- Kruanak K, Jarusutthirak C. Degradation of 2,4,6-trichlorophenol in synthetic wastewater by catalytic ozonation using alumina supported nickel oxides. J Environ Chem Eng 2019; 7(1):102825. doi: 10.1016/j.jece.2018.102825 [Crossref] [ Google Scholar]
- Moussavi G, Mahmoudi M. Degradation and biodegradability improvement of the Methylene Bluered 198 azo dye using catalytic ozonation with MgO nanocrystals. Chem Eng J 2009; 152(1):1-7. [ Google Scholar]
- Zhou L, Zhang S, Li Z, Liang X, Zhang Z, Liu R. Efficient degradation of phenol in aqueous solution by catalytic ozonation over MgO/AC. J Water Process Eng 2020; 36:101168. doi: 10.1016/j.jwpe.2020.101168 [Crossref] [ Google Scholar]
- Alkurdi SSA, Al-Juboori RA, Bundschuh J, Bowtell L, McKnight S. Effect of pyrolysis conditions on bone char characterization and its ability for arsenic and fluoride removal. Environ Pollut 2020; 262:114221. doi: 10.1016/j.envpol.2020.114221 [Crossref] [ Google Scholar]
- Rojas-Mayorga CK, Bonilla-Petriciolet A, Sánchez-Ruiz FJ, Moreno-Pérez J, Reynel-Ávila HE, Aguayo-Villarreal IA. Breakthrough curve modeling of liquid-phase adsorption of fluoride ions on aluminum-doped bone char using micro-columns: Effectiveness of data fitting approaches. J Mol Liq 2015; 208:114-21. doi: 10.1016/j.molliq.2015.04.045 [Crossref] [ Google Scholar]
- Rojas-Mayorga CK, Bonilla-Petriciolet A, Silvestre-Albero J, Aguayo-Villarreal IA, Mendoza-Castillo DI. Physico-chemical characterization of metal-doped bone chars and their adsorption behavior for water defluoridation. Appl Surf Sci 2015; 355:748-60. doi: 10.1016/j.apsusc.2015.07.163 [Crossref] [ Google Scholar]
- Mohammadi L, Rahdar A, Bazrafshan E, Dahmardeh H, Thysiadou A, Kyzas GZ. Benzene removal from aqueous solutions by heterogeneous catalytic ozonation process with magnesium oxide nanoparticles. Ozone Sci Eng. 2020:1-16. 10.1080/01919512.2020.1765738.
- Asgari G, Feradmal J, Poormohammadi A, Sadrnourmohamadi M, Akbari S. Taguchi optimization for the removal of high concentrations of phenol from saline wastewater using electro-Fenton process. Desalin Water Treat 2016; 57(56):27331-8. doi: 10.1080/19443994.2016.1170635 [Crossref] [ Google Scholar]
- Seid-Mohammadi A, Asgarai G, Ghorbanian Z, Dargahi A. The removal of cephalexin antibiotic in aqueous solutions by ultrasonic waves/hydrogen peroxide/nickel oxide nanoparticles (US/H2O2/NiO) hybrid process. Sep Sci Technol 2020; 55(8):1558-68. doi: 10.1080/01496395.2019.1603241 [Crossref] [ Google Scholar]
- Asgari G, Almasi H, Fardmal J, Ghanbari F, Daraie Z, Akbari S. Optimization of catalytic ozonation process for removal of reactive black 5 dye using bone char ash modified by magnesium oxide and applying taguchi design. Journal of Mazandaran University of Medical Sciences 2015; 24(122):252-62. [ Google Scholar]
- APHA, AWWA, WEF. Standard Methods for the Examination of water and wastewater. 21st ed. Washington, DC: APHA; 2005. p. 201.
- Saltuk Pirgalıoğlu, Tülay A. ÖzbelgeComparison of non-catalytic and catalytic ozonation processes of three different aqueous single dye solutions with respect to powder copper sulfide catalyst. Applied Catalysis A: General 2009; 363(1–2):157-163. doi: 10.1016/j.apcata.2009.05.011 [Crossref] [ Google Scholar]
- Sui M, Xing S, Sheng L, Huang S, Guo H. Heterogeneous catalytic ozonation of ciprofloxacin in water with carbon nanotube supported manganese oxides as catalyst. J Hazard Mater 2012; 227-228:227-36. doi: 10.1016/j.jhazmat.2012.05.039 [Crossref] [ Google Scholar]
- Valdés H, Farfán VJ, Manoli JA, Zaror CA. Catalytic ozone aqueous decomposition promoted by natural zeolite and volcanic sand. J Hazard Mater 2009; 165(1-3):915-22. doi: 10.1016/j.jhazmat.2008.10.093 [Crossref] [ Google Scholar]
- Asgari G, Seid-Mohammadi A, Mortazavi SB, Ramavandi B. Investigation on the pyrolysis of cow bone as a catalyst for ozone aqueous decomposition: kinetic approach. J Anal Appl Pyrolysis 2013; 99:149-54. doi: 10.1016/j.jaap.2012.10.008 [Crossref] [ Google Scholar]
- Moussavi G, Khavanin A, Alizadeh R. The investigation of catalytic ozonation and integrated catalytic ozonation/biological processes for the removal of phenol from saline wastewaters. J Hazard Mater 2009; 171(1-3):175-81. doi: 10.1016/j.jhazmat.2009.05.113 [Crossref] [ Google Scholar]
- Sousa HR, Silva LS, Sousa PAA, Sousa RRM, Fonseca MG, Osajima JA. Evaluation of methylene blue removal by plasma activated palygorskites. J Mater Res Technol 2019; 8(6):5432-42. doi: 10.1016/j.jmrt.2019.09.011 [Crossref] [ Google Scholar]
- El Hassani K, Kalnina D, Turks M, Beakou BH, Anouar A. Enhanced degradation of an azo dye by catalytic ozonation over Ni-containing layered double hydroxide nanocatalyst. Sep Purif Technol 2019; 210:764-74. doi: 10.1016/j.seppur.2018.08.074 [Crossref] [ Google Scholar]
- Subramani AK, Byrappa K, Ananda S, Lokanatha Rai KM, Ranganathaiah C, Yoshimura M. Photocatalytic degradation of indigo carmine dye using TiO2 impregnated activated carbon. Bull Mater Sci 2007; 30(1):37-41. doi: 10.1007/s12034-007-0007-8 [Crossref] [ Google Scholar]