Avicenna J Environ Health Eng. 7(1):1-7.
doi: 10.34172/ajehe.2020.01
Original Article
Toxicological Effects of Silver Nanoparticles on Nile Tilapia (Oreochromis niloticus)
Farahnaz Kakavand 1, Aliakbar Hedayati 1, *  , Ali Jafar Nodeh 1, Saeid Maddah 2, Maryam Rezaei Shadegan 1
, Ali Jafar Nodeh 1, Saeid Maddah 2, Maryam Rezaei Shadegan 1
Author information:
1Faculty of Fisheries and Environmental Sciences, Gorgan University of Agricultural Sciences and Natural Resources, Gorgan, Iran.
2Faculty of Environmental Sciences, Tehran University, Tehran, Iran.
Abstract
The wide use of nano-sized metallic materials could result in the release of these particles into the environment. In addition, due to the dissolution of these nano-materials, some of the adverse effects could result from the dissolved metals. On the other hand, dietary supplements play a key role in improving the immunity of consumers; hence, the use of immune stimulants such as mushroom seems to be very necessary. In this study, the dietary effect of Pleurotus ostreatus mushroom was investigated on serum immunity and liver histopathological indices of Oreochromis niloticus exposed to silver nanoparticles (AgNPs). Serum total protein level enhanced with increasing prebiotic concentration. Serum immunoglobulin and albumin levels increased in the group treated with Pleurotus ostreatus. The amount of these indices significantly increased at the concentration of 0.2 mg/kg; however, glucose value decreased in both of the experimental groups. Serum ALT and ALP levels significantly reduced in the combined treatment group (AgNPs and mushroom) at the concentration of 0.2 mg/kg, in contrast with the control group. In the combination treatment group (AgNPs and mushroom), the serum AST level significantly reduced at concentrations of 0.1 mg/kg and 0.2 mg/kg; however, it increased at the concentration of 0.05 mg/kg compared with the control group, indicating that immunological indices could improve due to the combined use of AgNPs and mushroom. The overall conclusion of this study shows that the use of mushroom at 0.2 mg/kg in combination with AgNPs could partially improve the effect of AgNPs on tilapia.
Keywords: Aquatic pollution, Resistance improvement, Metal nanoparticles, Pleurotus ostreatus
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
Prohibition and limitation of antibiotics as feed additives in fish culture in many countries have caused an increase in the number of studies on alternative dietary supplements such as probiotics and prebiotics to increase the health of cultured fish (1). Prebiotics are described as non-digestible food components that beneficially affect the host by selectively stimulating the growth and activity of bacteria in the intestinal tract (1). Generally, medicinal herbs are considered as potential immune enhancers and antioxidants when used in the land-based captive, hatchery and farmed animals (2,3). Over the past years, the use of prebiotics and other additives that have been instrumental in enhancing consumer safety has increased, and the positive effects of these substances in a variety of organisms have been proven. Dietary additives can affect fish physiology by elevating immune responses and health condition, improving growth rate, and also protecting fish against harmful agents (4).
The rapid improvement of nanotechnology has led to concerns about the health hazards and environmental effects of nanoparticles (NPs) (5). Potential toxic effects of NPs on aquatic environment and organisms have drawn more particular attention due to their high level of production, use, and inadvertent release into the environment (6). NPs are determined as materials with two or three dimensions between 1 and 100 nm. NPs show unique properties depending on their form, size, surface, construction, and physicochemical attributes (7). The special properties of silver nanoparticles (AgNPs) such as good conductivity, catalytic activities and antibacterial properties have made them the largest and most important kind of nanomaterial for commercial purposes (8). The production of nanomaterial and the release of NPs into the environment now appears inevitable since a growing number of products comprising these materials are quickly marketed and used worldwide (9), indicating that both aquatic and terrestrial habitats will be exposed to NPs (10). Ecotoxicology of nanomaterial products has become a newly emerging research field (11).
Histopathology is a simple and suitable way of measuring the adverse effects of pollutants on the structure of different tissues. Serum biochemical and histopathological parameters analyzed in the current study are generally admitted as powerful stress indicators and also are the main biomarkers of the health status of fish (12).
Nile tilapia (Oreochromis niloticus) is one of the most common aquaculture species in the world, because of the ease of breeding, high tolerance to different parameters and challenges, fast growth, and high market request (13). Given the increasing concern about the safety of NPs and their toxicity, along with all the beneficial aspects of NPs, their risk to living organisms also has to be noted and potential harmful effects should not be ignored by observing some of the achievements of nanotechnology. Therefore, the effect of prebiotics on immunological indices of aquatic animals may be beneficial in fish culture (1). In the present study, the effect of silver NPs on Nile tilapia fish (Oreochromis niloticus ) has been investigated and the hypothesis that silver NPs can be affected by supplementation of Pleurotus ostreatusmushroom will be proved.
2. Materials and Methods
The study was conducted for 42 days in Fisheries Research Center, Gorgan University of Agricultural Sciences and Natural Resources. This experiment was accomplished in a completely randomized design. At first, 120 Nile tilapia fry with a mean weight of 20 g were obtained from private reproduction centers. They were kept in adaptation tanks for one week for adaptation to the test environment. During the test period, the physicochemical factors of water were as follows: water temperature: 25-28°C, pH: 7.9-7.6, dissolved oxygen concentration: 7-9 mg/L, and water hardness: 210 mg/L calcium carbonate. After acclimatization, 10 fish were stored in 100-L fiberglass tanks. The mushroom (Pleurotus ostreatus) was purchased from a reputable manufacturer of mushrooms and was added to the base diet after being dried and powdered. The experiment was carried out with 4 treatments and each treatment with 3 replications including: commercial diet (treatment 1), diet containing 0.05 mg/kg of mushroom powder (treatment 2), diet containing 0.1 mg/kg of mushroom powder (treatment 3) and diet containing 0.2 mg/kg (food to body weight ratio) of mushroom powder (treatment 4). The fish were fed daily at 3 mg/kg of body weight and twice daily with experimental diets for 42 days. Based on the 96-hour LC50 values, fish were exposed to 50 mg/kg of silver nano-particles (Ag-NPs) (0/5 ppm) for 16 days (14).
At the end of the experiment (after 65 days), 9 blood samples were taken from each case. Fish were not fed 24 hours before sampling. Blood samples were obtained from the fish using a syringe (from the stem vein) (15). In order to prepare the serum, blood samples were centrifuged in a refrigerated centrifuge at 3000 rpm for 6 minutes at 4°C. Then, serum was isolated and transferred to the laboratory for measurement of serum biochemical parameters including hepatic enzymes aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), glucose (GLU), total protein (TP), albumin (Alb), and immunoglobulin (Ig). Blood biochemical parameters were measured using commercial kits (Pars Azmon, Iran) (16) and an autoanalyzer (Eppendorf, EPOS, Germany). Glucose levels were measured by the glucose oxidase methods (16).
Data analysis was carried out using one-way ANOVA and Duncan’s multiple range tests. Statistical analysis was carried out using SPSS software version 20.0 at a significant level of P < 0.05. The results were expressed as mean ± standard deviation.
3. Results and Discussion
All the experimental treatments had significant effects on all serum indices (P≥ 0.05). Serum total protein enhanced with increasing prebiotic concentration, and the value of this index increased in groups fed with both mushroom and AgNPs, at concentrations of 0.05 mg/kg and 0.2 mg/kg. Serum Ig increased in the group treated with Pleurotus ostreatus, the value of this index significantly increased in the group treated with 0.2 mg/kg of mushroom. Serum Ig levels significantly increased in the combined treatment group (AgNPs and mushroom) at a concentration of 0.2 mg/kg. Serum albumin level in the mushroom-fed group increased with the concentration of mushroom; in addition, it increased in the combined treatment group (AgNPs and mushroom) at concentrations of 0.1 mg/kg and 0.2 mg/kg.
The glucose value decreased in both of the experimental groups as compared with the control, this index significantly decreased in the combined treatment group at concentrations of 0.1 mg/kg and 0.2 mg/kg groups (Fig. 1).
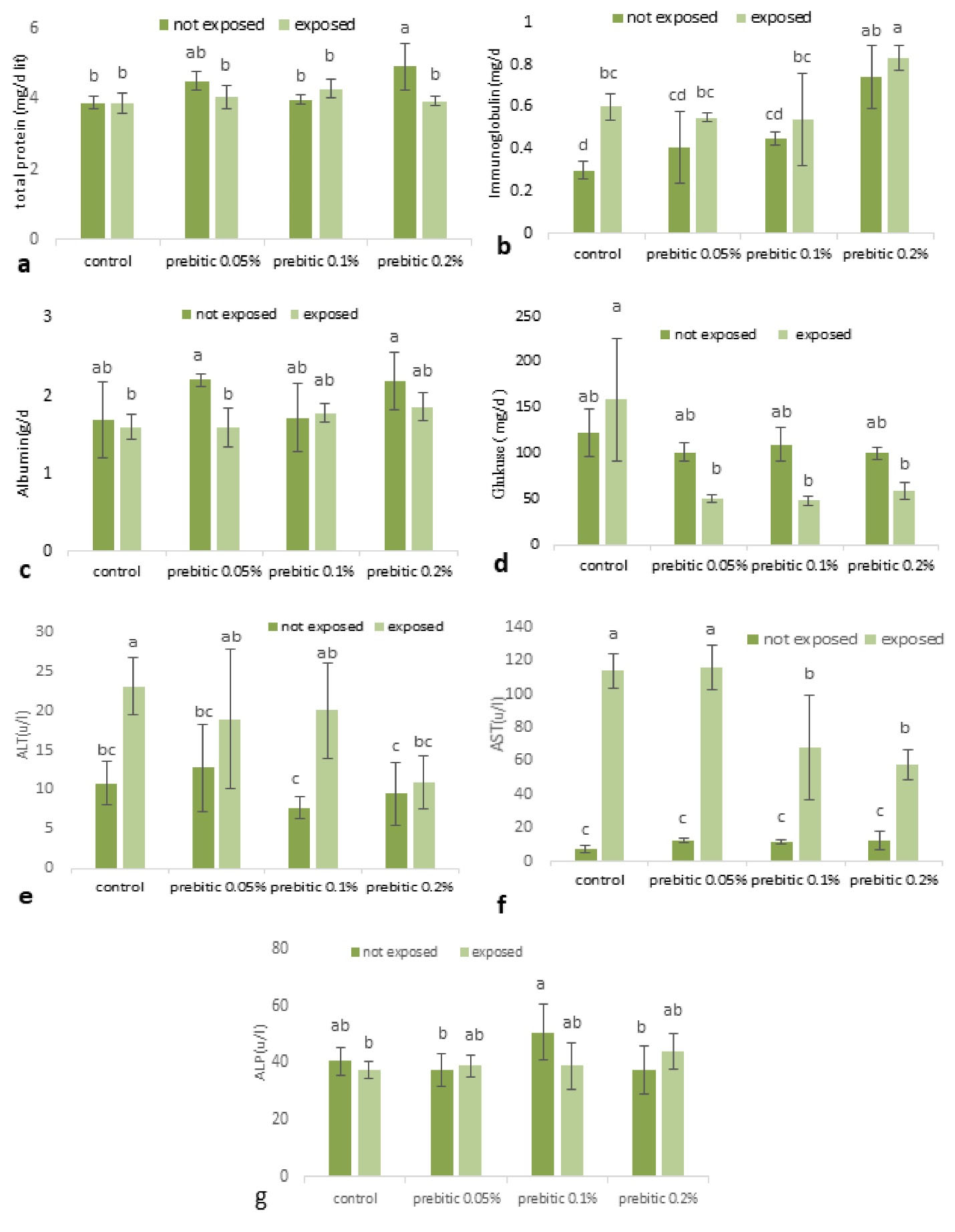
Figure 1.
Liver Histopathological Indices of Tilapia Exposed to AgNPs (a) Total Protein, (b) Immunoglobulin, (c) Albumin, (d) Glucose, (e) ALT, (f) AST, and (g) ALP.
.
Liver Histopathological Indices of Tilapia Exposed to AgNPs (a) Total Protein, (b) Immunoglobulin, (c) Albumin, (d) Glucose, (e) ALT, (f) AST, and (g) ALP.
Serum ALT significantly reduced in the combined treatment group (AgNPs and mushroom) at the concentration of 0.2 mg/kg, in contrast with the control group. In the group treated with AgNPs and Mushroom, the level of this index was lower compared to the control group, indicating that mushroom alone led to a decrease in ALT but it was not able to neutralize or improve the effect of AgNPs. In the combined treatment group (AgNPs and Mushroom), the level of serum AST significantly reduced at 0.1 mg/kg and 0.2 mg/kg; however, it increased at 0.05 mg/kg compared with the control group. Serum ALP decreased in mushroom-fed groups at concentrations of 0.05 mg/kg and 0.2 mg/kg; however, the amount of this index enhanced with the combination treatment of AgNPs and mushroom in comparison with the control group, indicating that mushroom could improve the effect of AgNPs (Fig. 1).
AgNP is a hazardous pollutant that has various toxic effects on aquatic animals. The biomagnifying effects of AgNPs on the food chain also pose challenges to the public health system. Fish indicators are dependent on a variety of factors such as species, size, age, physiological status, environmental conditions, and diet (quantity and quality of food, dietary ingredients, protein sources, vitamins, and growth promoters) (17). It seems that hematological indices, biochemical changes, growth rates, and oxygen consumption of fish can be used to determine the toxicity of pollutants. Due to the association of the circulatory system and the external environment, biochemical indices of the blood can be used to detect the effects of stress and toxic substances. Changes in biochemical indices and destruction of blood-forming organs in fish may be due to environmental conditions or contamination of water. This study investigated the use of diet containing Pleurotus ostreatus mushroom for protection against waterborne exposure to AgNPs in fish. The plant extracts exert known and proven effects on the immune system in different organisms. This effect is due to the host non-specific immunogenicity effects on hemorrhagic immunity or cellular immunity. Until now, many studies have been carried out on the promotion of immunity in humans through the use of various immunostimulants, in which plants are recommended due to their proper effect and lack of adverse effects (18).
The most abundant proteins found in plasma are albumins and globulins. The main function of these proteins is the regulation of osmotic pressure of blood, the transfer of internal and external compounds such as free fatty acids, hormones, and drugs. Serum proteins have the highest immune effect on serum Ig. Moreover, the level of Ig significantly enhanced not only in group treated with AgNPs but also in the group treated with mushroom; it means that mushroom improved the effect of AgNPs when combined with it at 0.05 mg/kg and 0.1 mg/kg. The increase of total protein levels in the serum is a good indicator for assessing the status of the immune system and fish health. Our data showed that each experimental treatment had a different effect on serum protein, Ig, albumin, and glucose levels. Plasma proteins have been studied in different types of organisms and are classified into different groups according to their functions (16). In a previous study, an increase was observed in total protein and albumin levels of Caspian salmon fry fed with nucleotide (19). Additionally, a significant increase was observed in serum protein levels of nucleotide-fed Catla(20). The evaluation of nutritional supplements showed that the Ig level significantly increased during exposure to silver NPs, which is similar to the results of this study.
Accordingly, the treatment conducted with silver NPs causes severe tissue damage such as Necrosis, Dilution, Dark granules, Inflation, Blood blockage, Bleeding and Bile stagnation and these effects have been greatly reduced by probiotic treatments and this reduction was more evident at the concentration of 2 mg/kg (Table 1, Fig. 2).
Table 1.
Histopathological Changes in the Liver of Nile Tilapia (Oreochromis niloticus ) Exposed to Concentrations of Silver NPs Combined with Pleurotus Ostreatus Mushroom
Histopathological
Effects
|
Toxin-Free Control Without Prebiotics
|
Toxin Control Without Prebiotics
|
Prebiotic
0.05 mg/kg
|
Prebiotic
0.1 mg/kg
|
Prebiotic
0.2 mg/kg
|
| Atrophy |
_ |
+ + + |
+ + + |
+ + |
+ + |
| Fatty |
+ |
+ + + |
+ + |
+ |
+ |
| Necrosis |
_ |
+ + + |
+ + |
+ |
+ |
| Dilution |
+ |
+ + + |
+ + |
+ + |
_ |
| Dark granules |
_ |
+ + |
+ + |
+ |
_ |
| Inflation |
_ |
+ + |
+ + |
+ + |
+ |
| Blood blockage |
_ |
+ + + |
+ + |
+ |
+ |
| Bleeding |
_ |
+ + + |
+ + |
_ |
_ |
| Bile stagnation |
_ |
_ |
_ |
_ |
_ |
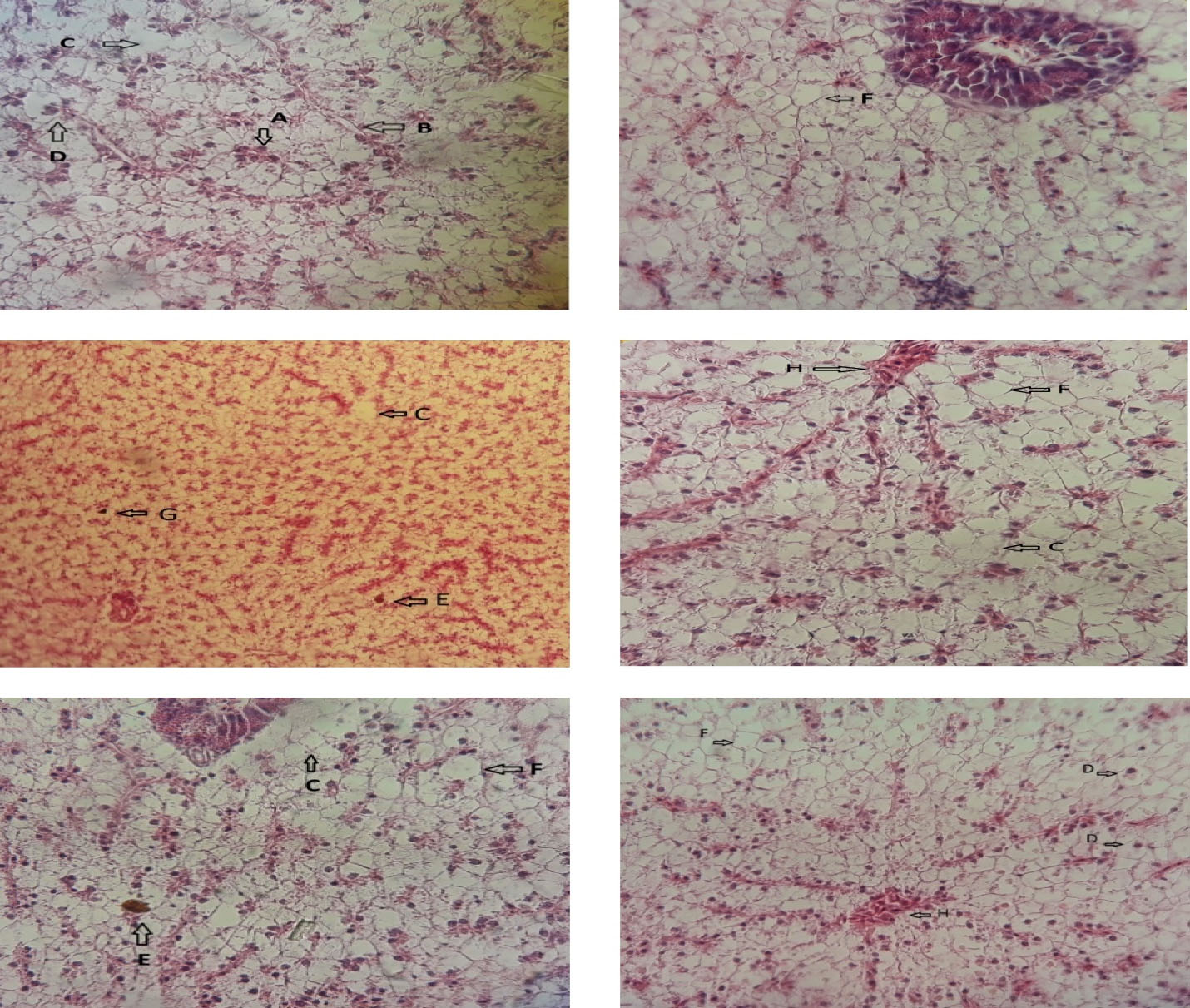
Figure 2.
Histopathological Lesions of Liver of Nile Tilapia (Oreochromis niloticus ) after Exposure to Different Concentrations of AgNPs; (A) Atrophy (B) Fat (C) Necrosis (D) dilution (F) Dark granules (E) Blood blockage (G) Bleeding (H) Bile Stagnation.
.
Histopathological Lesions of Liver of Nile Tilapia (Oreochromis niloticus ) after Exposure to Different Concentrations of AgNPs; (A) Atrophy (B) Fat (C) Necrosis (D) dilution (F) Dark granules (E) Blood blockage (G) Bleeding (H) Bile Stagnation.
Blood glucose is a highly variable parameter that is strongly influenced by environmental stresses and manipulations such as seasonal variations, nutritional status, and sexual maturity. The level of glucose significantly decreased in the group treated with AgNPs. Further, mushroom in combination with AgNPs could improve the effect of AgNPs.Liver enzymes are considered to be indicators of liver activity, and changes in levels of activity and secretion can be influenced by physical and chemical factors of water, concentration, breeding conditions, type of diet, age, sex, and health status of fish.
The metal pollutants can increase or decrease the activity of liver enzymes and changes in liver histopathology. Given that the liver is the main place for biological alterations and it protects the body from foreign materials and xenobiotic chemicals, recognizing the reasons for hepatic toxicity is of great importance. The liver excretes the materials into bile; as a result, the biliary organism is also exposed to NPs. Previous studies have shown that different toxins with various mechanisms, including including activation of alcohol decadence, membrane lipid peroxidation, inhibition of protein synthesis, disturbance of calcium homeostasis, and activation of receptor enzymes, cause damage to the liver cells (7).
In this research, nanosilver treatments insignificantly increased ALP level (P<0.05). In the combined treatment, there was not any significant impact on the ALP. The alkaline phosphatase enzyme acts as an antibacterial agent due to its hydrolytic activity in serum, and its level could increase during the early stages of wound healing, stress conditions due to the protective role against pathogens (21) and the use of immunostimulants in the diet (22). ALP is a key enzyme in liver function, since the liver is the organ primarily responsible for the metabolism of non-biotic materials by changing the morphological structure of these materials. ALT and AST are liver enzymes that indicate damage to liver cells. The amount of these enzymes in liver cells depends on many factors, meaning that when the hepatic cells and membrane are damaged, the value of these enzymes increases in the bloodstream and this increase in value is a symptom of the liver damage (23). For this reason, liver damage evaluations have been widely used to measure levels of enzymes such as ALT, AST, and ALP. The incidence of necrosis or damage to the membrane of the cell causes the release of these enzymes into the circulation. Enhanced levels of serum AST cause liver damage, such as viral hepatitis, myocardial infarction, and muscle damage. ALT, which catalyzes the transformation of alanine into pyruvate and glutamate, is more specific to the liver and is a more appropriate parameter for detecting liver damage. Increased levels of serum enzymes indicate cellular leakage, structural damage, and dysfunction of the cell membranes in the liver. One of the reasons for increasing the serum level of these enzymes may be the change in the permeability of the plasma membrane of the liver cells or the cellular damage caused by exposure to NPs. Therefore, monitoring leakage of liver enzymes into the bloodstream is a very useful tool for investigating liver damage caused by NPs (24).
The effect of iron oxide NPs on liver tissue and enzymes showed that high concentrations of iron oxide NPs could have adverse effects on the liver, damage liver tissue and increase the level of liver enzymes. Further studies show that iron oxide NPs at concentrations above 150 mg/kg increase the ALT level in Rainbow trout. This enzyme is specific to the liver and damage to the liver cells leads to increased release of this enzyme. Hence, the reason for the increase of this enzyme may be the destructive effect of iron oxide NPs on liver cells. Aminotransferases serve as markers of the health of the liver cells, and in the early stages of liver degeneration, these cytoplasmic enzymes of hepatocytes leak from the cell membranes into the bloodstream and the permeability of the membrane increases. These enzymes are likely to be released into the blood due to the loss of liver cells. Therefore, an increase in these enzymes is a sign of damage to liver cells (25). In general, the difference in the results of other researchers depends on several factors because the serum parameters are affected by a large number of internal and external factors such as species, water temperature, reproductive cycle, metabolic rate, age, stress, light periods, nutritional status, and method of determination.
4. Conclusion
According to the results of the present study, the use of Pleurotus ostreatus mushroom, which is a kind of plant extract, could control the immune system in fish, especially when treated with higher concentrations. The overall conclusion of this study implied that the level of Ig significantly increased in both Ag-NPs and mushroom treatment groups, which means that mushroom improved the effect of AgNPs when combined with AgNPs at concentrations of 0.05 mg/kg and 0.1 mg/kg. ALT and AST levels also increased extremely in the AgNPs treatment group. However, the Mushroom did not have any effect on these two types of enzymes. The use of mushroom (0.2 mg/kg) in combination with AgNPs could improve the effect of AgNPs.Additionally, the amount of glucose significantly decreased in AgNPs treatment group. In summary, the AgNPs had a toxic effect on the indices including ALT, AST, and Ig in Cyprinus carpio. The Mushroom separately had also a significant effect on the Ig and total protein. The supplementation of the diet with mushroom improves the physiologic indices including ALT, AST, and Ig, with the best function at the 2 mg/kg. Furthermore, the use of 2 mg/kg of mushroom in the diet is suggested to increase the resistance of fish to the toxicity of AgNPs.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgments
This research was conducted with the support of Gorgan University of Agricultural Sciences and Natural Resources as an MSc thesis. The authors would like to thank all those who contributed to this research.
References
- Ringø E, Olsen RE, Gifstad TØ, Dalmo RA, Amlund H, Hemre GI. Prebiotics in aquaculture: a review. Aquac Nutr 2010; 16(2):117-36. doi: 10.1111/j.1365-2095.2009.00731.x [Crossref] [ Google Scholar]
- El-Deep MH, Dawood MAO, Assar MH, Ijiri D, Ohtsuka A. Dietary Moringa oleifera improves growth performance, oxidative status, and immune related gene expression in broilers under normal and high temperature conditions. J Therm Biol 2019; 82:157-63. doi: 10.1016/j.jtherbio.2019.04.016 [Crossref] [ Google Scholar]
- Saleh AA, Kirrella AA, Dawood MAO, Ebeid TA. Effect of dietary inclusion of cumin seed oil on the performance, egg quality, immune response and ovarian development in laying hens under high ambient temperature. J Anim Physiol Anim Nutr (Berl) 2019; 103(6):1810-7. doi: 10.1111/jpn.13206 [Crossref] [ Google Scholar]
- Dawood MAO, Koshio S, Esteban MÁ. Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquac 2018; 10(4):950-74. doi: 10.1111/raq.12209 [Crossref] [ Google Scholar]
- Zhu X, Zhou J, Cai Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar Pollut Bull 2011; 63(5-12):334-8. doi: 10.1016/j.marpolbul.2011.03.006 [Crossref] [ Google Scholar]
- Blaise C, Gagné F, Férard JF, Eullaffroy P. Ecotoxicity of selected nano-materials to aquatic organisms. Environ Toxicol 2008; 23(5):591-8. doi: 10.1002/tox.20402 [Crossref] [ Google Scholar]
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 2005; 113(7):823-39. doi: 10.1289/ehp.7339 [Crossref] [ Google Scholar]
- Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol 2008; 42(11):4133-9. doi: 10.1021/es7032718 [Crossref] [ Google Scholar]
- Aitken RJ, Chaudhry MQ, Boxall AB, Hull M. Manufacture and use of nanomaterials: current status in the UK and global trends. Occup Med (Lond) 2006; 56(5):300-6. doi: 10.1093/occmed/kql051 [Crossref] [ Google Scholar]
- Handy RD, Shaw BJ. Toxic effects of nanoparticles and nanomaterials: implications for public health, risk assessment and the public perception of nanotechnology. Health Risk Soc 2007; 9(2):125-44. doi: 10.1080/13698570701306807 [Crossref] [ Google Scholar]
- Handy RD, Owen R, Valsami-Jones E. The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs. Ecotoxicology 2008; 17(5):315-25. doi: 10.1007/s10646-008-0206-0 [Crossref] [ Google Scholar]
- Hussain R, Mahmood F, Khan A, Javed MT, Rehan S, Mehdi T. Cellular and biochemical effects induced by atrazine on blood of male Japanese quail (Coturnix japonica). Pestic Biochem Physiol 2012; 103(1):38-42. doi: 10.1016/j.pestbp.2012.03.001 [Crossref] [ Google Scholar]
- Dawood MAO, Magouz FI, Salem MFI, Abdel-Daim HA. Modulation of digestive enzyme activity, blood health, oxidative responses and growth-related gene expression in GIFT by heat-killed Lactobacillus plantarum (L-137). Aquaculture 2019; 505:127-36. doi: 10.1016/j.aquaculture.2019.02.053 [Crossref] [ Google Scholar]
- Handy RD, von der Kammer F, Lead JR, Hassellöv M, Owen R, Crane M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 2008; 17(4):287-314. doi: 10.1007/s10646-008-0199-8 [Crossref] [ Google Scholar]
- Fiess JC, Kunkel-Patterson A, Mathias L, Riley LG, Yancey PH, Hirano T. Effects of environmental salinity and temperature on osmoregulatory ability, organic osmolytes, and plasma hormone profiles in the Mozambique tilapia (Oreochromis mossambicus). Comp Biochem Physiol A Mol Integr Physiol 2007; 146(2):252-64. doi: 10.1016/j.cbpa.2006.10.027 [Crossref] [ Google Scholar]
- Abedian Kenari A, Mahmoudi N, Soltani M, Abediankenari S. Dietary nucleotide supplements influence the growth, haemato-immunological parameters and stress responses in endangered Caspian brown trout (Salmo trutta caspius Kessler, 1877). Aquac Nutr 2013; 19(1):54-63. doi: 10.1111/j.1365-2095.2012.00938.x [Crossref] [ Google Scholar]
- Lim C, Klesius PH, Li MH, Robinson EH. Interaction between dietary levels of iron and vitamin C on growth, hematology, immune response and resistance of channel catfish (Ictalurus punctatus) to Edwardsiella ictaluri challenge. Aquaculture 2000; 185(3-4):313-27. doi: 10.1016/s0044-8486(99)00352-x [Crossref] [ Google Scholar]
- Ghasemi-Pirbaloti A, Pirali A, Pishkar GH, Jalali MA, Reyesi M, Jafarian M. Effect of some essential extract of some medicinal plants on the systemicity of Rainbow trout. Journal of Herbal Drugs 2011; 2(2):155-149. [ Google Scholar]
- Jha AK, Pal AK, Sahu NP, Kumar S, Mukherjee SC. Haemato-immunological responses to dietary yeast RNA, omega-3 fatty acid and beta-carotene in Catla catla juveniles. Fish Shellfish Immunol 2007; 23(5):917-27. doi: 10.1016/j.fsi.2007.01.011 [Crossref] [ Google Scholar]
- Khaleghi SR, Hedayati SAA, Kashiri H, Paknejad H, Hosseinifar SH. Effect of Pediococcus acidilactici probiotic and Agaricus bisporus prebiotic supplements on the expression of immune-related genes in common carp (Cyprinus carpio) exposed to silver nanoparticles. Aquatics Physiology and Biotechnology 2019; 6(4):155-74. doi: 10.22124/japb.2019.9818.1233.[Persian] [Crossref] [ Google Scholar]
- Palaksha KJ, Shin GW, Kim YR, Jung TS. Evaluation of non-specific immune components from the skin mucus of olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol 2008; 24(4):479-88. doi: 10.1016/j.fsi.2008.01.005 [Crossref] [ Google Scholar]
- Roosta Z, Hajimoradloo A, Ghorbani R, Hoseinifar SH. The effects of dietary vitamin C on mucosal immune responses and growth performance in Caspian roach (Rutilus rutilus caspicus) fry. Fish Physiol Biochem 2014; 40(5):1601-7. doi: 10.1007/s10695-014-9951-6 [Crossref] [ Google Scholar]
- Dambach DM, Andrews BA, Moulin F. New technologies and screening strategies for hepatotoxicity: use of in vitro models. Toxicol Pathol 2005; 33(1):17-26. doi: 10.1080/01926230590522284 [Crossref] [ Google Scholar]
- Park EJ, Bae E, Yi J, Kim Y, Choi K, Lee SH. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ Toxicol Pharmacol 2010; 30(2):162-8. doi: 10.1016/j.etap.2010.05.004 [Crossref] [ Google Scholar]
- Christ-Crain M, Meier C, Puder J, Staub JJ, Huber PR, Keller U. Changes in liver function correlate with the improvement of lipid profile after restoration of euthyroidism in patients with subclinical hypothyroidism. EXCLI J 2004; 3(1):1-9. doi: 10.17877/de290r-14916 [Crossref] [ Google Scholar]