Avicenna J Environ Health Eng. 11(1):39-46.
doi: 10.34172/ajehe.5404
Original Article
Evaluating the Influence of Environmental Factors on the Stability of Commercial Chlorine-Based Sanitizers Using Response Surface Modeling
Mohammad Ali Zazouli 1  , Yalda Hashempour 2, *
, Yalda Hashempour 2, *  , Nafiseh Dashtban 3, *
, Nafiseh Dashtban 3, * 
Author information:
1Department of Environmental Health Engineering, Health Sciences Research Center, Faculty of Health, Mazandaran University of Medical Sciences, Sari, Iran
2Department of Environmental Health Engineering, Faculty of Health, Mazandaran University of Medical Sciences, Sari, Iran
3Department of Environmental Health Engineering, Faculty of Health, Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran
Abstract
Given the increasing use of chlorine-based disinfectants and concerns regarding their shelflife and persistence on the surface, the present study investigated the effect of environmental conditions on the stability of commercial solutions stored under different conditions. This study assessed the stability of 0.05%, 0.5%, and 1% sodium hypochlorite solutions at pH levels of 5, 7.5, and 10 stored at temperatures of 4 ° C, 25 ° C, and 45 ° C using iodometric titration at 0, 15, 30, and 60 days after preparation. Eighty-seven solutions from three commercial brands (Brand A, Brand B, and Brand C) were prepared based on experimental design and response surface modeling (RSM) using Box-Behnken factorial design (BBD). The results revealed that the linear coefficient effects of all factors (temperature, storage time, pH, and initial concentration) were statistically significant (P<0.05). However, no significant differences were found among the three commercial brands (P=0.307). A negative correlation was observed between free available chlorine (FAC) and storage time. Solutions with a higher initial concentration (1%) tended to have lower stability. Higher temperatures also contributed to the instability of the solutions. Moreover, a gradual decrease was observed in the pH over time, and the lowest stability was detected at a neutral pH of 7.5. These findings suggest that environmental conditions (e.g., temperature, storage duration, pH, and initial concentration) significantly affect the stability of chlorine in the solutions. Therefore, these factors should be considered when using chlorine and storing chlorine-based disinfectants (CBDs) to ensure optimal performance and effectiveness.
Keywords: Stability, Environmental factors, Sodium hypochlorite, Response surface methodology,
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Zazouli MA, Hashempour Y, Dashtban N. Evaluating the influence of environmental factors on the stability of commercial chlorine-based sanitizers using response surface modeling. Avicenna J Environ Health Eng. 2024; 11(1):39-46. doi:10.34172/ajehe.5404
1. Introduction
Disinfectants are chemical substances specifically designed to deactivate or kill microorganisms on inert surfaces or living tissues. These compounds include different categories such as acids, alkalis, alcohols, phenols, aldehydes, halogens, detergents, biguanides, and quaternary ammonium compounds (1). Chlorine remains one of the most widely used disinfectants globally, and the high oxidizing and disinfecting characteristics of chlorine were identified in the early 19th century (2,3).
Hypochlorite, the simplest chlorine compound, is the most useful chemical in the laboratory and industry (4). Sodium hypochlorite (NaOCl or NaClO), commonly referred to as bleach, is a vital disinfectant and a strong oxidizing agent. The two major sources of NaOCl are pharmaceutical supplies and commercial household bleaches with 3%-6% available chlorine (5-7). Commercial laundry bleaches contain 12%-15% available chlorine. The decomposition of chlorine solutions highly depends on their concentration, pH, and temperature (8). The percentage concentration of NaOCl in aqueous solutions (HOCl and OCl forms) is determined based on the weight per volume of free available chlorine (FAC) (9,10). Available chlorine levels are important factors affecting the activity of NaOCl solutions, which can degrade overtime due to exposure to various environmental factors such as light, heat, air, metal ions, and organic substances (11,12). This has raised concerns about the chemical stability of the chlorine-containing solutions after opening the bottle. Consequently, it is often recommended to prepare fresh solutions at the time of use(4,13). Al-Gheethi et al also suggest that diluted chlorine should be freshly prepared (13).
However, existing studies have shown that chlorine solutions are stable for extended periods under various storage conditions. The research by Pişkin and Türkün (4), e Silva Leonardo et al (14), Iqbal et al (8), van der Waal et al (9), and Rutala et al (15) have reported varying stabilization times for chlorine solutions.
There are several approaches to quantify available chlorine, including tissue solubility, N-diethyl-p-phenylenediamine ferrous ammonium sulfate analysis, titration to sodium arsenite, and iodometric titration with various endpoint detection methods, including titration to sodium thiosulfate and amperometric titration to phenylarsine (11).
The outbreak of the COVID-19 pandemic has significantly increased the use of chlorine-based disinfectants (CBDs). Chlorine sanitizers are extensively applied in hospitals, quarantine facilities, homes, laboratories, and public areas to combat the spread of the coronavirus, and their effectiveness against the virus on various surfaces has been well-documented (16,17). However, the use of CBDs is accompanied by challenges, including direct health issues such as respiratory problems, skin irritation, and mental health problems, as well as indirect health problems such as the formation of disinfection by-products and ecosystem disruption (16).
Response surface modeling (RSM) is a collection of statistical and mathematical techniques, which is typically used to design experiments for efficient and effective data collection (18,19). Common experimental designs include central composite designs, Box-Behnken factorial designs (BBDs), and factorial designs. The collected data are then used to fit a mathematical model, usually a polynomial model, describing the relationship between the response variable and the input variables (20).
In this context, the main challenge is understanding the shelf-life and stability of chlorine solutions under different conditions. The novelty of this study lies in its comprehensive investigation of the stability of three commercially available chlorine-based sanitizers (Brand A, Brand B, and Brand C) under various environmental conditions, including temperature, storage time, pH, and concentration. The study aimed to provide valuable insights into the stability of chlorine solutions and their available chlorine concentrations under different conditions, which is particularly relevant given the increased use of chlorine sanitizers during the COVID-19 pandemic. Previous studies have explored the stability of chlorine solutions, but this study offers a more detailed and comparative analysis of multiple commercial products under a wider range of conditions using RSM. The findings of this study will contribute to a better understanding of the shelf-life and practical application of CBDs, addressing the challenges and questions surrounding their storage and usage.
2. Material and Methods
2.1. Solutions
Three different commercial chlorine-based sanitizer brands were used as the sources of NaOCl for the current study: Brand A bleaching liquid (Golrang Industrial, Tehran, Iran), Brand B bleaching liquid (Active Industrial, Tehran, Iran), and Brand C bleaching liquid (Tage Industrial, Qazvin, Iran). All commercial solutions contained 5% FAC. Solutions with different concentrations (0.05%, 0.5%, and 1% w/v) were prepared using sterile distilled water (without chlorine).
2.2. Experimental Design
The study utilized the RSM to evaluate the factors affecting the stability of chlorine in the solutions. Specifically, a BBD was employed. The BBD is a three-level experimental design, with the input variables (temperature, contact time, pH, initial concentration of chlorinated solutions, and disinfection types) coded as -1, 0, and + 1. In each experiment, changes are made in the input variables to determine the causes of changes in the response variable (the stability of chlorinated solutions or their chlorine content) (21). The experimental design contained 87 runs, with each run performed in duplicate. The lower and upper limits of the variable levels are presented in Table 1.
Table 1.
Specifications of the Independent Variables Used in BBD
|
Input Factors
|
Units
|
-1
|
0
|
+1
|
| Temperature (X1) |
°C |
4 |
25 |
45 |
| Time (X2) |
Day |
15 |
30 |
60 |
| pH (X3) |
- |
5 |
7.5 |
10 |
| The initial concentration of disinfectants (X4) |
% |
0.05 |
0.5 |
1 |
| Disinfectant type (X5) |
- |
Brand A |
Brand B |
Brand C |
Note. BBD: Box–Behnken factorial design.
The following equation was used to code the five quantitative independent variables:
where Xj and Xi are the coded and actual values of an independent variable, respectively. X0 is the actual value of an independent variable at the center point, and ∆Xi is the step change value (21,22). The stability of chlorinated solutions was modeled using the following equation:
Eq. (2)
where Y is the predicted response variable, and β0, βi, βii, and βij are the constant regression coefficients of the model. The R2 and F-value are used to determine the accuracy and fitness of the model. The predicted values for the stability of chlorinated solutions were obtained by Design Expert software (version 11, Stat-Ease). Moreover, the analysis of variance (ANOVA) test was performed to check the fitness of the developed model equation.
2.3. pH Measurement
The pH of the solutions was adjusted to 5, 7.5, and 10 using sodium hydroxide and sulfuric acid. The pH of the solutions was measured and recorded after each experimental step using a digital pH meter (EUTECH INSTRUMENTS, CyberScan pH 5500, Netherlands).
2.4. Determination of the Available Chlorine Content
The available chlorine concentrations of all solutions were assessed immediately after preparation. The solutions were stored in matte plastic bottles wrapped in aluminum foil (to protect from light), with minimal headspace between the solution and the bottle cap. The solutions were stored at 4 °C (refrigerator), 25 °C (room temperature), and 45 °C (incubator) for 15, 30, and 60 days, respectively. At the end of each experimental run, the percentage of available chlorine was determined using iodometric titration, in according with the ASTM D2022 standard (23).
3. Results and Discussion
Considering the growing use of CBDs and the questions surrounding their shelf-life, persistence on the surface, and other environmental factors, the present study employed ESM to investigate the stability of commercial chlorinated solutions stored under various conditions.
3.1. Box–Behnken Design Matrix
A multiple regression analysis was applied to the data, as described in Eq. (2), to obtain the predicted response Y for the stability of the chlorinated solutions. In the Design Expert software, the square root of the data was used to normalize the data obtained from the tests.
The final equation in terms of actual factors for three commercial disinfectants is as follows:
For Brand A:
Eq. (3)
For Brand B:
Eq. (4)
For Brand C:
Eq. (5)
The coefficients obtained from Eq. (3), Eq. (4), and Eq. (5) are the square root of the data. To find the real coefficients, these coefficients must be raised to power 2. All data obtained from the three equations are statistically significant (P < 0.05). T-test, F-value, P value, mean square, and the sum of squares (SSs) were applied to assess the significance of each coefficient of the equations (Table 2). A higher F-value and SS indicate a higher validity of the model. An F-value of 28.08, P˂ 0.0001, and SS of 1142.18 suggest that the model is significant for the stability of chlorine in commercial solutions. The non-significant lack of fit (F-value of 0.80) implies that the lack of fit is not significant relative to the pure error, indicating a 62.75% chance that a lack of fit F-value this large could occur due to noise.
Table 2.
ANOVA, Regression Coefficient Estimate, and Test of Significance for the Stability of Chlorinated Solutions (Response Surface Quadratic Model)
|
Factor
|
Sum of Squares
|
Mean Squares
|
Coefficient Estimated±SE
|
df
|
F
-value
|
Probability* (
P
)>
F
|
| Intercept (Model) |
1142.18 |
47.59 |
8.74 ± 0.3362 |
24 |
28.08 |
< 0.0001 |
| X1 |
83.2 |
83.2 |
1.52 ± 0.217 |
1 |
49.08 |
< 0.0001 |
| X2 |
21.12 |
21.12 |
0.7659 ± 0.217 |
1 |
12.46 |
0.0008 |
| X3 |
175.01 |
175.01 |
-2.2 ± 0.217 |
1 |
103.24 |
< 0.0001 |
| X4 |
145.85 |
145.85 |
2.01 ± 0.217 |
1 |
86.04 |
< 0.0001 |
| X1X4 |
15.76 |
15.76 |
-1.15 ± 0.3758 |
1 |
9.29 |
0.0034 |
| X2X3 |
6.83 |
6.83 |
-0.7544 ± 0.3758 |
1 |
4.03 |
0.0491 |
| X2X4 |
16.36 |
16.36 |
-1.17 ± 0.3069 |
1 |
9.65 |
0.0029 |
| X3X4 |
36.24 |
36.24 |
-1.74 ± 0.3069 |
1 |
21.38 |
< 0.0001 |
| X12 |
19.47 |
19.47 |
1 ± 0.2951 |
1 |
11.49 |
0.0012 |
| X32 |
519.21 |
519.21 |
-5.17 ± 0.2951 |
1 |
306.31 |
< 0.0001 |
| X42 |
63.47 |
63.47 |
-1.81 ± 0.2951 |
1 |
37.44 |
< 0.0001 |
| Residual |
105.09 |
1.7 |
|
62 |
|
|
| Lack of fit |
81.10 |
1.66 |
|
49 |
0.80 |
0.6275 |
Note. ANOVA: Analysis of variance; SE: Standard error, df: Degree of freedom. *This table appropriately includes only the significant parameter estimates. A non-significant lack of fit indicates a good model fit.
The ANOVA for the response surface quadratic model revealed that the linear effects of temperature (X1, P < 0.0001), time (X2, P < 0.0001), pH (X3, P = 0.0008), and final chlorine concentration (X4, P < 0.0001) are significant. There were no statistically significant differences between the three brands, introduced with X5 in Table 2 (P = 0.307). However, the interactive effects of X1X4 (P = 0.0034), X2X3 (P = 0.0491), X2X4 (P = 0.0029), and X3X4 (P < 0.0001) were significant. The quadratic terms X12 (P = 0.0012),X32 (P < 0.0001),and X42 (P < 0.0001) were also significant.
Focusing on the model that maximizes the adjusted R2 and the predicted R2, the quadratic model achieved the highest values, with a predicted R2 of 0.8135 and an adjusted R2 of 0.8831. The signal-to-noise (S/N) ratio was 18.95, demonstrating a sufficient signal for the model, as a ratio greater than 4 indicates a good fit.
Fig. 1 shows the interaction effect of temperature and concentration on the stability efficiency of chlorine-based solutions for Brand A, Brand B, and Brand C. The data suggest that as the concentration increases from 0.05% to 1%, the stability decreases for all commercial solutions. Additionally, stability efficiency was maximum at 4°C, but it decreased with an increase in temperature up to 45°C. The Brand C shows the lowest stability efficiency, while the Brand A and Brand B exhibit similar stability.
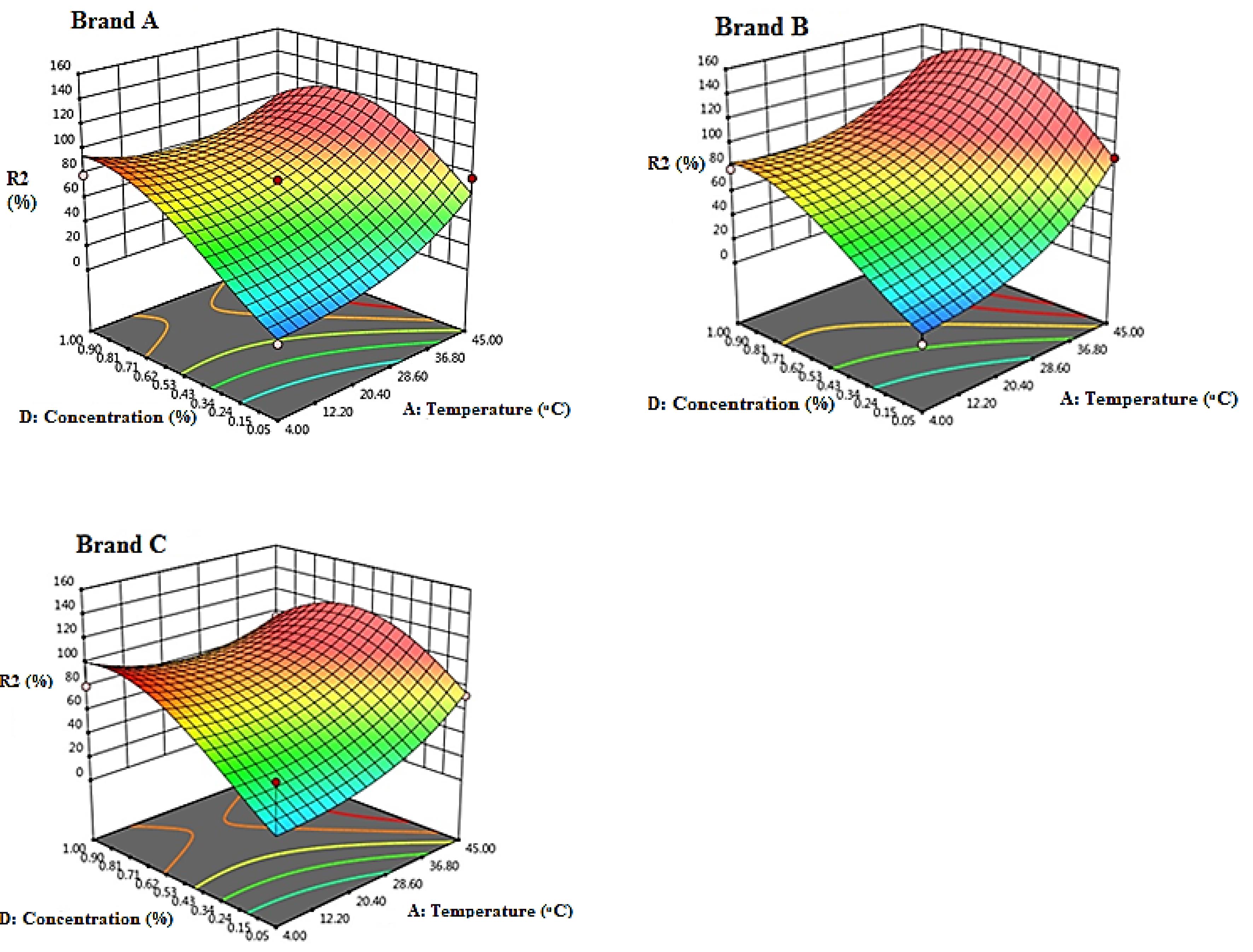
Fig. 1.
Response Surface 3D Plots Showing the Effect of Temperature and Initial Concentration of Chlorinated Solutions (%) on the Stability of Chlorine-based Solutions (pH = 7.5, Time = 30 days) (Brand A = Golrang, Brand B = Active, Brand C = Tage)
.
Response Surface 3D Plots Showing the Effect of Temperature and Initial Concentration of Chlorinated Solutions (%) on the Stability of Chlorine-based Solutions (pH = 7.5, Time = 30 days) (Brand A = Golrang, Brand B = Active, Brand C = Tage)
Fig. 2 illustrates the stability efficiency of chlorine-based solutions as a result of the interaction between time and concentration. The highest stability was observed at 15 days, and the stability of the solutions decreased over time from 15 to 60 days. Based on this, the order of stability of the solutions is Brand A ˃ Brand B ˃ Brand C.
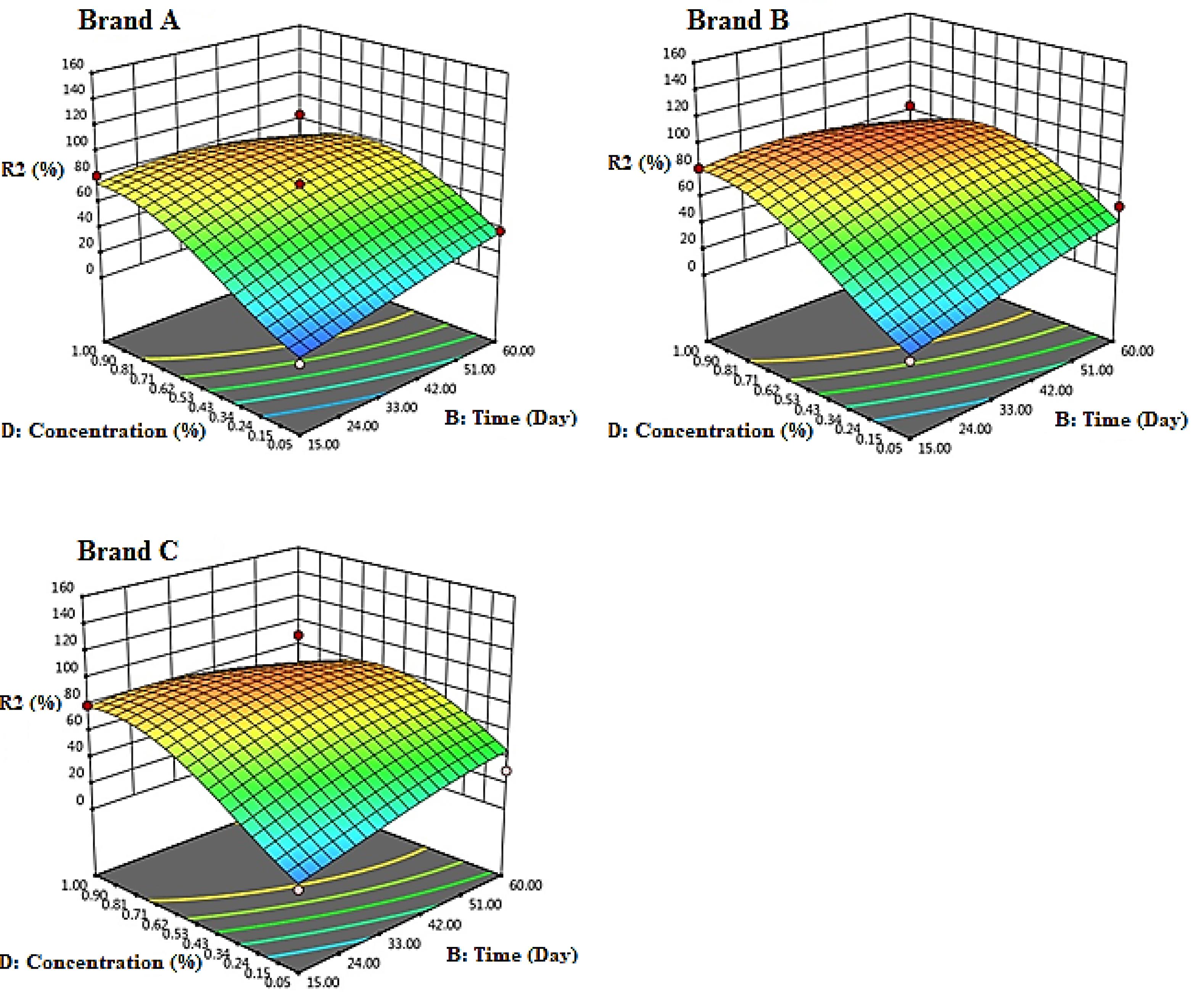
Fig. 2.
Response Surface 3D Plots Showing the Effect of Time and Initial Concentration of Chlorinated Solutions (%) on the Stability of Chlorine-Based Solutions (Temperature = 25°C, pH = 7.5)(Brand A = Golrang, Brand B = Active, Brand C = Tage)
.
Response Surface 3D Plots Showing the Effect of Time and Initial Concentration of Chlorinated Solutions (%) on the Stability of Chlorine-Based Solutions (Temperature = 25°C, pH = 7.5)(Brand A = Golrang, Brand B = Active, Brand C = Tage)
Fig. 3 presents the stability efficiency of chlorine-based solutions as a result of the interaction between pH and concentration. The highest stability was observed in acidic and alkaline pH conditions, while the stability was minimal at pH 7.5. Furthermore, there was no significant difference between the three brands of chlorinated solutions.
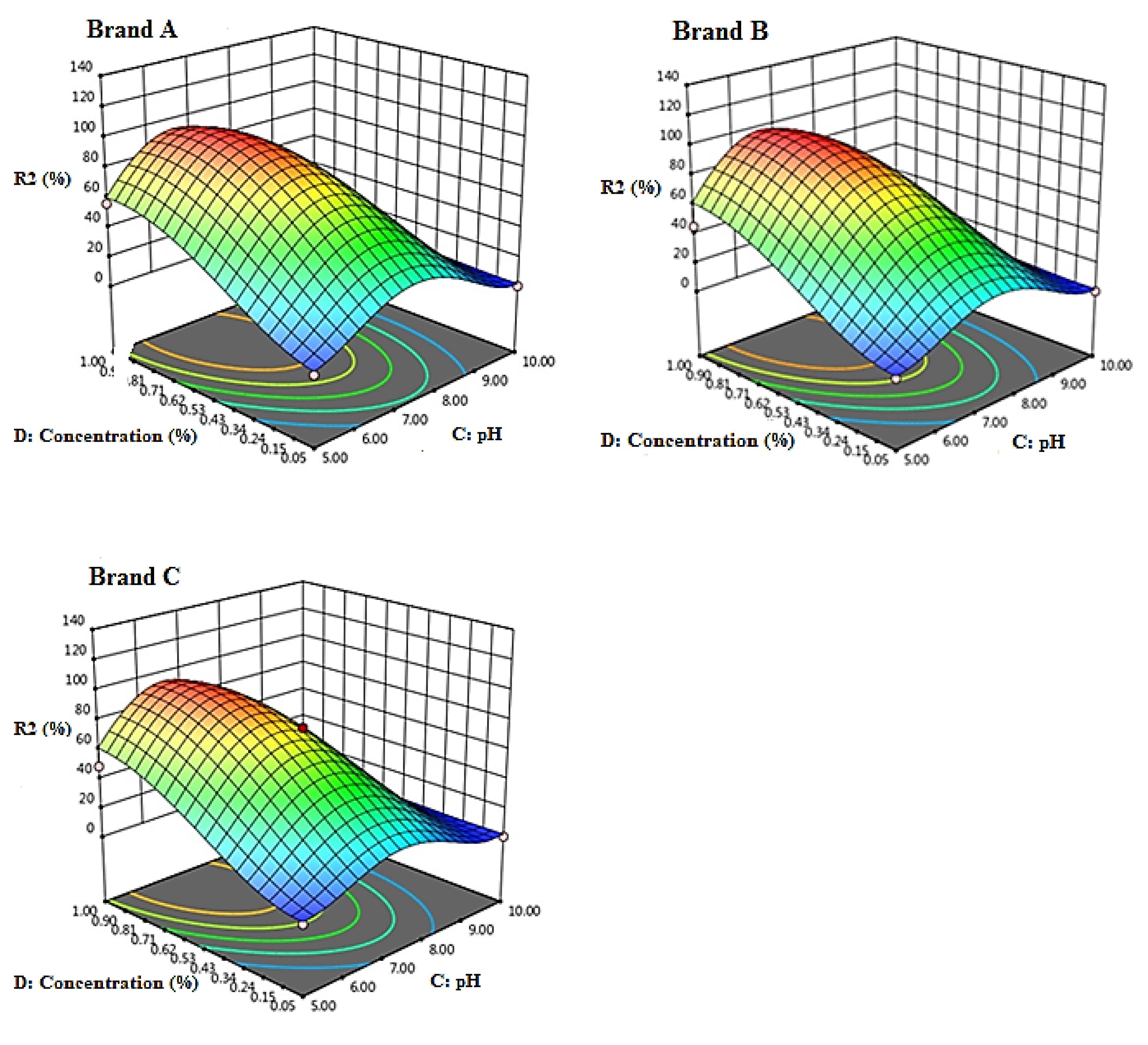
Fig. 3.
Response Surface 3D Plots Showing the Effect of pH and Initial Concentration of Chlorinated Solutions (%) on the Stability of Chlorine-Based Solutions (Temperature = 25°C, Time = 30 days) (Brand A = Golrang, Brand B = Active, Brand C = Tage)
.
Response Surface 3D Plots Showing the Effect of pH and Initial Concentration of Chlorinated Solutions (%) on the Stability of Chlorine-Based Solutions (Temperature = 25°C, Time = 30 days) (Brand A = Golrang, Brand B = Active, Brand C = Tage)
In sum, all factors (temperature, storage time, pH, and initial chlorine concentration) have affected the stability of chlorine in solutions. Additionally, there was no significant difference in available chlorine between the three different brands of commercial chlorine-based sanitizers.
3.2. The Effect of Temperature on the Stability of Chlorine Solutions
Temperature is a critical factor that affects the stability of NaOCl solutions. The results of this study indicate that as temperature increased, the stability of the chlorine solutions decreased for all examined commercial brands. This finding is consistent with the observations reported by Iqbal et al, suggesting a maximum shelf-life of 30 days for NaOCl solutions stored at 35 °C (8). Similarly, the study by e Silva Leonardo et al found that both NaOCl and calcium hypochlorite solutions, predominantly at concentrations of 0.5% and 1%, lose their stability under the influence of increased temperature (14). The underlying reason for this phenomenon is that as the temperature rises, the active chlorine in the solutions, which contribute to the antimicrobial effect, is released more rapidly (12,24). This accelerates the degradation of chlorinated solutions, as demonstrated in the study conducted by Frais et al, showing that continuous and prolonged exposure to heat can accelerate the degradation of chlorinated solutions and reduce their effectiveness during extended storage periods (5).
In summary, the present study aligns with the existing literature, confirming that temperature is a critical factor in the stability of CBD solutions. As the temperature increases, the stability and, consequently, the antimicrobial efficacy of these solutions tend to decrease over time. This understanding is crucial for the proper storage and handling of chlorinated disinfectants to ensure their long-term effectiveness.
3.3. The Effect of Storage Time on the Stability of Chlorine Solutions
The current study measured the FAC in the chlorine-based solutions at 15, 30, and 60 days after preparation using iodometric titration. The results indicated that the FAC of all the solutions decreases over time. Specifically, the available chlorine of solutions after 60 days was lower than those stored for 15 and 30 days, suggesting a decrease in the stability of the solutions over time. These findings align with the observations reported by Iqbal et al who found that at a temperature of 35 °C, the suggested maximum shelf-life for Ca(OCl)2, NaOCl, and NaDCC was 30 days, 4-6 days, and 2 days, respectively (8). This highlights the importance of temperature as a critical factor in the stability of CBDs.
Additionally, the results are consistent with the findings of Guastalli et al who reported a substantial decrease in the FAC in the early steps of the analyses, followed by a more gradual decrease over a longer period (25). This pattern of decreasing FAC over time has been observed in several previous investigations as well (12,15,26).
The consistent decrease in the FAC of the stored chlorine-based solutions across multiple studies underscores the importance of understanding the factors that influence the stability of these disinfectants. This knowledge is crucial for ensuring the effective and safe use of chlorine-based products as the reduced FAC can compromise their antimicrobial efficacy over time. In conclusion, the current study’s findings, consistent with the existing literature, demonstrate that the FAC in chlorine-based solutions decreases over time, highlighting the need for careful storage conditions and proper handling to maintain the stability and effectiveness of these disinfectants.
3.4. The Effect of pH on the Stability of Chlorine Solutions
The results of the current study indicated a gradual decrease in the pH of all stored solutions under different conditions over time. This observation aligns with the findings of a study conducted by Camps et al, which demonstrated a reduction in the pH of a 10% NaOCl solution from 7.3 to 6.4 during the first two hours, followed by a more rapid decrease to 9 over the next 3 hours (27). Two possible explanations can be considered for this pH change. Firstly, the amount of free NaOH in a 10% NaOCl solution is extremely low, so a small amount of acid can easily neutralize the existing OH ions. Secondly, HOCl, which is the dominant species in the 2.5% NaOCl, can readily decompose to Cl2, which has low solubility in water, and then rapidly decomposes to Cl. This process does not apply to the H+ ions, which accelerate the drop in pH. Additionally, the presence of carbon dioxide in the air can dissolve into the solution and form carbonic acid, further contributing to the pH decline (27). These combined factors can describe the drop in pH after five hours and the resulting low levels of available chlorine. These findings contrast with the results of studies by Johnson and Remeikus (28), Fabian and Walker (29), and Clarkson et al (11), suggesting that alkaline NaOCl solutions can be relatively stable for extended periods (weeks or months), even when exposed to light. However, these studies recommend using the test liquid within the first two hours after mixing.
According to the findings of the current study, the decomposition rates of NaOCl solutions with pH of 5 and 10 were lower, indicating better stability under acidic and alkaline conditions. In contrast, the lowest stability was detected at neutral pH (7.5) for all three commercial brands of solutions.
These findings are in contrast with the study by Pişkin and Türkün (4), which reported that the pH of investigated NaOCl solutions does not affect their stability. However, the results of the study by. e Silva Leonardo et al (14) support the current findings. They showed that NaOCl can ionize in an aqueous solution, releasingHOCl and OH ions. The OCl- ion is the predominant species at alkaline pH, while HOCl is predominant at acidic pH. Both of these ions play a crucial role in the availability of free chlorine in a solution. The OCl- acts as a reservoir for the formation of HOCl, and when it is consumed, a decrease in pH occurs, increasing the chlorine release and altering the shelf-life.
Furthermore, a study by Johnson and Remeikus (28) identified a gradual reduction in pH of 5.25% NaOCl to 1% NaOCl solutions over time, which supports the findings of the current study.
3.5. The Effect of Initial Concentration on the Stability of Chlorine Solutions
Another significant factor in the stability of chlorinated solutions is the initial concentration of these solutions. According to the results of this study, as the initial concentration of solution increased, the stability efficiency decreased. This is in contrast with the findings of Clarkson et al who reported that highly concentrated solutions are more stable over time compared to less concentrated solutions (11). However, the current findings are consistent with the study by Pişkin and Türkün who reported that the available chlorine in 2% NaOCl decreases by 34% after 200 days at room temperature (4). Similarly, Frais et al demonstrated that the available chlorine in 15% NaOCl decreases by 14.1% after 81 days (5).
4. Conclusion
The results of the RSM using BBD in Design Expert software revealed that the linear coefficient effects of temperature, storage time, pH, and initial concentration are significant (P < 0.05). Additionally, the interactive effects of temperature-concentration, time-pH, time-concentration, and pH-concentration were also significant. The ANOVA for the quadratic RSM showed that the experiments are highly accurate, and the model is very reliable, indicating a good fit of the model equation. Importantly, the type of commercial chlorine-based sanitizers (Brand A, Brand B, and Brand C) under study did not exhibit any significant difference in terms of stability. To conclude, all three brands of commercial chlorine-based sanitizers displayed similar stability profiles. Increasing temperature and storage time led to a decrease in the stability of solutions. Interestingly, solutions with neutral pH (7.5) were more stable compared to acidic and alkaline solutions. Furthermore, increasing the initial concentration of the solution resulted in enhanced stability. These findings highlight the importance of considering factors such as temperature, storage duration, pH, and initial concentration when formulating and storing chlorine-based sanitizers to ensure their optimal stability and effectiveness. The insights gained from this study can inform the development and quality control of commercial CBD products.
Acknowledgements
The authors would like to express their gratitude to the staff at the Mazandaran University of Medical Sciences for the financial support (grant number IR.MAZUMS.REC.1400.486).
Authors’ Contribution
Conceptualization: Mohammad Ali Zazouli.
Data curation: Nafiseh Dashtban.
Formal analysis: Yalda Hashempour.
Funding acquisition: Mohammad Ali Zazouli.
Investigation: Mohammad Ali Zazouli, Yalda Hashempour.
Methodology: Yalda Hashempour.
Project administration: Yalda Hashempour.
Resources: Mohammad Ali Zazouli.
Software: Yalda Hashempour.
Supervision: Mohammad Ali Zazouli.
Validation: Mohammad Ali Zazouli, Yalda Hashempour.
Visualization: Mohammad Ali Zazouli, Yalda Hashempour.
Writing–original draft: Nafiseh Dashtban.
Writing–review & editing: Mohammad Ali Zazouli, Yalda Hashempour.
Competing Interests
The authors declare no conflicts of interests.
Ethical Approval
This study was conducted in accordance with ethical standards and received approval from the Mazandaran University of Medical Sciences Ethics Committee, under ethical code IR.MAZUMS.REC.1400.486.
Funding
This research was funded by Mazandaran University of Medical Sciences with grant number IR.MAZUMS.REC.1400.486.
References
- Dhama K, Patel SK, Kumar R, Masand R, Rana J, Yatoo MI. The role of disinfectants and sanitizers during COVID-19 pandemic: advantages and deleterious effects on humans and the environment. Environ Sci Pollut Res Int 2021; 28(26):34211-28. doi: 10.1007/s11356-021-14429-w [Crossref] [ Google Scholar]
- Aali R, Kishipour A. Risk Assessment of Drinking Water Supply System of Talesh Based on World Health Organization Water Safety Plan in 2021: A Case Study. Avicenna J Environ Health Eng 2022; 9(1):54-61. doi: 10.34172/ajehe.2022.07 [Crossref] [ Google Scholar]
- Brinkmann MT, Rong K, Xie Y, Yan T. Formation potential of disinfection byproducts during chlorination of petroleum hydrocarbon-contaminated drinking water. Chemosphere 2024; 357:142057. doi: 10.1016/j.chemosphere.2024.142057 [Crossref] [ Google Scholar]
- Pişkin B, Türkün M. Stability of various sodium hypochlorite solutions. J Endod 1995; 21(5):253-5. doi: 10.1016/s0099-2399(06)80991-x [Crossref] [ Google Scholar]
- Frais S, Ng YL, Gulabivala K. Some factors affecting the concentration of available chlorine in commercial sources of sodium hypochlorite. Int Endod J 2001; 34(3):206-15. doi: 10.1046/j.1365-2591.2001.00371.x [Crossref] [ Google Scholar]
- World Health Organization (WHO). Cleaning and Disinfection of Environmental Surfaces in the Context of COVID-19. WHO; 2020.
- Slaughter RJ, Watts M, Vale JA, Grieve JR, Schep LJ. The clinical toxicology of sodium hypochlorite. Clin Toxicol (Phila) 2019; 57(5):303-11. doi: 10.1080/15563650.2018.1543889 [Crossref] [ Google Scholar]
- Iqbal Q, Lubeck-Schricker M, Wells E, Wolfe MK, Lantagne D. Shelf-life of chlorine solutions recommended in Ebola virus disease response. PLoS One 2016; 11(5):e0156136. doi: 10.1371/journal.pone.0156136 [Crossref] [ Google Scholar]
- van der Waal SV, van Dusseldorp NE, de Soet JJ. An evaluation of the accuracy of labeling of percent sodium hypochlorite on various commercial and professional sources: is sodium hypochlorite from these sources equally suitable for endodontic irrigation?. J Endod 2014; 40(12):2049-52. doi: 10.1016/j.joen.2014.08.021 [Crossref] [ Google Scholar]
- Clarkson RM, Podlich HM, Moule AJ. Influence of ethylenediaminetetraacetic acid on the active chlorine content of sodium hypochlorite solutions when mixed in various proportions. J Endod 2011; 37(4):538-43. doi: 10.1016/j.joen.2011.01.018 [Crossref] [ Google Scholar]
- Clarkson RM, Moule AJ, Podlich HM. The shelf-life of sodium hypochlorite irrigating solutions. Aust Dent J 2001; 46(4):269-76. doi: 10.1111/j.1834-7819.2001.tb00291.x [Crossref] [ Google Scholar]
- Gambarini G, De Luca M, Gerosa R. Chemical stability of heated sodium hypochlorite endodontic irrigants. J Endod 1998; 24(6):432-4. doi: 10.1016/s0099-2399(98)80027-7 [Crossref] [ Google Scholar]
- Al-Gheethi A, Al-Sahari M, Abdul Malek M, Noman E, Al-Maqtari Q, Mohamed R. Disinfection methods and survival of SARS-CoV-2 in the environment and contaminated materials: a bibliometric analysis. Sustainability 2020; 12(18):7378. doi: 10.3390/su12187378 [Crossref] [ Google Scholar]
- e Silva Leonardo NG, Carlotto IB, Luisi SB, Kopper PM, Grecca FS, Montagner F. Calcium hypochlorite solutions: evaluation of surface tension and effect of different storage conditions and time periods over pH and available chlorine content. J Endod 2016; 42(4):641-5. doi: 10.1016/j.joen.2016.01.006 [Crossref] [ Google Scholar]
- Rutala WA, Cole EC, Thomann CA, Weber DJ. Stability and bactericidal activity of chlorine solutions. Infect Control Hosp Epidemiol 1998; 19(5):323-7. doi: 10.1086/647822 [Crossref] [ Google Scholar]
- Parveen N, Chowdhury S, Goel S. Environmental impacts of the widespread use of chlorine-based disinfectants during the COVID-19 pandemic. Environ Sci Pollut Res Int 2022; 29(57):85742-60. doi: 10.1007/s11356-021-18316-2 [Crossref] [ Google Scholar]
- Rahmani AR, Azarian G, Poormohammadi A. Health impacts of long-term exposure to disinfectants during SARS-Cov-2 pandemic. Avicenna J Environ Health Eng 2020; 7(1):53-4. doi: 10.34172/ajehe.2020.08 [Crossref] [ Google Scholar]
- Abdul Aziz H, Syed Zainal SF, Alazaiza MY. Optimization of coagulation-flocculation process of landfill leachate by Tin (IV) chloride using response surface methodology. Avicenna J Environ Health Eng 2019; 6(1):41-8. doi: 10.34172/ajehe.2019.06 [Crossref] [ Google Scholar]
- Ghasemi Z, Mir Bagheri SA, Vafaei F. Optimization of a two-stage reverse osmosis pilot system for Caspian seawater desalination using response surface methodology: SEC reduction through recovery increase, Fe fouling, and scaling control. Int J Environ Sci Technol 2024; 21(1):493-514. doi: 10.1007/s13762-023-05277-x [Crossref] [ Google Scholar]
- Buenaño L, Ali E, Jafer A, Zaki SH, Hammady FJ, Khayoun Alsaadi SB. Optimization by Box-Behnken design for environmental contaminants removal using magnetic nanocomposite. Sci Rep 2024; 14(1):6950. doi: 10.1038/s41598-024-57616-8 [Crossref] [ Google Scholar]
- Yazdanbakhsh A, Hashempour Y, Ghaderpouri M. Performance of granular activated carbon/nanoscale zero-valent iron for removal of humic substances from aqueous solution based on experimental design and response surface modeling. Glob Nest J 2018; 20(1):57-68. [ Google Scholar]
- Majlesi M, Hashempour Y. Removal of 4-chlorophenol from aqueous solution by granular activated carbon/nanoscale zero valent iron based on response surface modeling. Arch Environ Prot 2017; 43(4):13-25. doi: 10.1515/aep-2017-0035 [Crossref] [ Google Scholar]
- ASTM. Standard Test Methods of Sampling and Chemical Analysis of Chlorine-Containing Bleaches. D20222017.
- Abou-Rass M, Oglesby SW. The effects of temperature, concentration, and tissue type on the solvent ability of sodium hypochlorite. J Endod 1981; 7(8):376-7. doi: 10.1016/S0099-2399(81)80059-3 [Crossref] [ Google Scholar]
- Guastalli AR, Clarkson RM, Rossi-Fedele G. The effect of surfactants on the stability of sodium hypochlorite preparations. J Endod 2015; 41(8):1344-8. doi: 10.1016/j.joen.2015.03.009 [Crossref] [ Google Scholar]
- Aparecida Nicoletti M, Fernandes Magalhäes J. [Influence of the container and environmental factors in the stability of sodium hypochlorite]. Bol Oficina Sanit Panam 1996;121(4):301-9. [Spanish].
- Camps J, Pommel L, Aubut V, Verhille B, Satoshi F, Lascola B. Shelf life, dissolving action, and antibacterial activity of a neutralized 25% sodium hypochlorite solution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 108(2):e66-73. doi: 10.1016/j.tripleo.2009.03.034 [Crossref] [ Google Scholar]
- Johnson BR, Remeikis NA. Effective shelf-life of prepared sodium hypochlorite solution. J Endod 1993; 19(1):40-3. doi: 10.1016/s0099-2399(06)81040-x [Crossref] [ Google Scholar]
- Fabian TM, Walker SE. Stability of sodium hypochlorite solutions. Am J Hosp Pharm 1982; 39(6):1016-7. [ Google Scholar]