Avicenna J Environ Health Eng. 11(1):27-32.
doi: 10.34172/ajehe.5323
Original Article
Air Pollution, Ozone, and Sulfur Dioxide Can Affect the Blood Serum Lipid Profile and Oxidative Stress of Male Wistar Rats
Hossein Mashhadi-Abdolahi 1  , Roya Darbani 2, Oldouz Rabet 2, Asal Golchin 3
, Roya Darbani 2, Oldouz Rabet 2, Asal Golchin 3  , Sorayya Kheirouri 4
, Sorayya Kheirouri 4  , Mohammad Alizadeh 5
, Mohammad Alizadeh 5  , Mehran Mesgari-Abbasi 2, *
, Mehran Mesgari-Abbasi 2, * 
Author information:
1Tabriz Health Services Management Research Center, Tabriz, Iran
2Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Biochemistry, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran
4Department of Nutrition, Tabriz University of Medical Sciences, Tabriz, Iran
5Nutrition Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
The majority of the body’s organs are impacted by ambient air pollution (AAP), which is currently a serious environmental global health issue. The objective of this work was to assess the impact of ozone (O3), sulfur dioxide (SO2), and AAP on oxidative stress (OS) and lipid profile indicators in male Wistar rats. To this end, these rats were exposed for three hours each day for five weeks in control, AAP, O3 (0.6 ppm), and SO2 (10 ppm) groups (each containing 8 animals). Several parameters, such as total antioxidant capacity (TAC), superoxide dismutase (SOD), glutathione peroxidase (GPx) activities, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride, and malondialdehyde (MDA), were measured on blood samples. AAP exposure on TAC (P=0.014) and SOD (P=0.05), MDA (P=0.018), and HDL (P=0.003), O3 exposure on TAC (P<0.001), and SO2 exposure on blood serum TAC (P=0.006) all had statistically significant effects. Based on the results, exposure to SO2 and AAP did not significantly alter the lipid profile (P>0.05). According to our research, exposure to O3, SO2, and AAP can increase overall antioxidant capacity by stimulating blood serum oxidative defense enzymes. Except for the enhanced effect of O3 exposure on serum HDL, AAP, SO2, and O3 exposures had no discernible effects on the lipid profile.
Keywords: Air pollution, Lipid profile, Oxidative stress, Ozone, Rats, Sulfur dioxide,
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1. Introduction
Every year, exposure to air pollutants, as an environmental health problem, results in 7 million fatalities worldwide and is regarded as a risk factor for non-communicable diseases (1). Particulate matter, ozone (O3), sulfur oxides (SO2), and nitrogen oxides (NOx) are the primary constituents (2). Particulate matter smaller than 2.5 microns (PM2.5) can cause lung cancer and chronic obstructive pulmonary and heart diseases by deeply penetrating the lung and affecting the vascular and respiratory systems (3,4). The population of industrialized and developing nations is impacted by ambient air pollution (AAP). Over 91% of people on the planet are thought to reside in areas with air quality levels higher than that described in the World Health Organization’s (WHO’s) guidelines (5). Nevertheless, developing nations such as those in Southeast Asia and the Western Pacific have the largest burden (6).
Evidence suggests that exposure to air pollution mostly affects the cardiovascular system, contrary to the previous assumption that it impacts respiratory diseases (7). There is evidence to support the hypothesis that oxidative stress (OS) modifies lipid metabolism and, in turn, the systemic inflammatory response (8). Triglycerides, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) are factors that make up lipid profiles. Their levels are impacted by AAP, which in turn helps initiate cardiovascular events.
Conflicting findings have been found despite research on the relationship between lipid profile measures or dyslipidemia and AAP exposure (9‒13). Although the lipid profile parameters were shown to be sensitive to AAP (14), some research found no correlation between AAP and TC and HDL levels (15).
The generation of free radicals may be facilitated by exposure to environmental elements such as radiation, O3, smoke from cigarettes, and air pollution (16). An imbalance between antioxidants and free radicals is known as OS (17). Antioxidants can keep free radicals in check when they are more prevalent (18). Free radicals can harm organic molecules such as proteins, lipids, and DNA. Over time, this damage can lead to problems in human organs, such as atherosclerosis (19), heart failure (20), myocardial infarction (21), and cancer (22).
While many countries have witnessed a decrease in air pollution in recent years, industrialization, especially in those with fossil fuel resources, implies that these endeavors will not, even in the medium run, solve pollution-related issues. Thus, a more focused strategy to remove the most harmful air pollutants and maybe a way to lessen individual influences and sensitivity to air pollutants is made possible by a more comprehensive understanding of the fundamental mechanisms behind health issues brought on by air pollution (23,24). Accordingly, this study aimed to evaluate the association between AAP, SO2, and O3 on the lipid profile, including TC, HDL-C, LDL-C, and TG, as well as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), malondialdehyde (MDA), and total antioxidant capacity (TAC) in male Wistar rats.
2. Materials and Methods
2.1. Animal Procedures
This study was conducted under approval by the Animal Research Ethics Committee located at Tabriz University of Medical Sciences under certificate No. IR.TBZMED.REC.1399.083.
Thirty-two 180‒220 g weighted Wistar male rats (Pasteur Institute, Iran) were placed at 20‒24 °C under 12/12-hour light/dark cycle conditions. The sample size was determined according to previous animal studies and the guidelines of the National Committee for Ethics in Biomedical Research on reducing the sample size of animal studies. The food and water were freely available. Before experimentation, the animals were acclimatized to the mentioned conditions for a week.
The animals were randomly divided into four groups (n = 8 in each group) as follows:
-
The Control Group: The rats in this group only had filtered air available to them. The air filtration of this group was performed using HEPA and activated carbon filters (Panam Azma, Iran). The control group’s levels of SO2, O3, and P2.5 contaminants were undetectable (25).
-
The SO2 Group: For five weeks, the rats were exposed to 26 mg/m3 (10 ppm) of SO2 three hours a day. A 40 x 50 x 60 cm³ hand-made glass enclosure with top and bottom vents were used as SO2 exposure chamber. The SO2 for the exposure chamber was supplied by two 50-liter SO2 cylinders operating at 120 bar pressure and 1 L/Min. The gases that made up the chamber were SO2 (0.002%), N (79.5%), and O2 (20.5%). A 2–30 ppm measurement range SO2 detector tube (GASTEC No. 5La) was used to check the SO2 concentration in the chamber on a daily basis. The air pressure was normal, and the temperature was 22 ± 1 °C (25).
-
The Ozone group: For five weeks, three hours a day, the rats in this group were exposed to O3 (1.18 ± 0.19 mg/m3, 0.6 ± 0.1 ppm) in a chamber house previously mentioned using an O3 generator system (Afra Sanat, Iran). A detector (Eco Sensors, model A-21ZX-USA) was utilized to measure the O3 content in the chamber daily (25).
-
The Ambient Air Pollution Group: The rats were placed in a high-traffic city square in the Tabriz city center (Abresan Square, Tabriz, East Azerbaijan, Iran) close to the air pollution recorder station for 3 hours a day for five weeks (25). The air pollutant mean concentrations for Tabriz Abresan Station during the study were extracted from Iranian AAP Monitoring System reports for Tabriz Abresan Station (Iranian Air Pollution Monitoring website, available at http://aqms.doe.ir/Home/AQI).
The rats were given ketamine (60 mg/kg IP) and xylazine (10 mg/kg IP) to induce unconsciousness twenty hours following the previous intervention, and blood samples were taken. The samples were put into tubes for biochemical analysis, and serum samples were isolated and kept in a freezer at -70°C (25).
2.2. Statistical Analyses
Data were analyzed using SPSS (Statistical Package for the Social Sciences) for Windows, version 19. The data between the groups were compared with a one-way analysis of variance, followed by Tukey’s post-hoc test. The overall significance of the study was assessed at P < 0.05.
3. Results and Discussion
3.1. Air Pollution Monitoring
The Iranian AAP Monitoring system reported the air pollutant mean concentrations for Tabriz Abresan Station during the study. According to the results, the mean ± standard deviation (SD) of the ambient air concentration was 2.00 ± 1.17 μg/m3, 14.08 ± 10.07, and 23.96 ± 8.26 for SO2, CO, and NO2, respectively. In terms of O3, it was 49.76 ± 18.48, 28.67 ± 4.04 for PM2.5, and 21.33 ± 5.13 μg/m3 for PM10 (Iranian Air Pollution Monitoring website, available at http://aqms.doe.ir/Home/AQI).
3.2. Alterations in Indicators of Oxidative Stress Following Exposure to Different Gases and Ambient Air Pollution
Figs. 1-3 show TAC, SOD, and MDA level changes between groups after exposure to different pollutants. As illustrated in Fig. 1, the TAC level increased in all exposure groups compared to the control. There were significant differences between the control group and SO2 group (P=0.006), O3 (P<0.001), and AAP (P=0.014) groups.
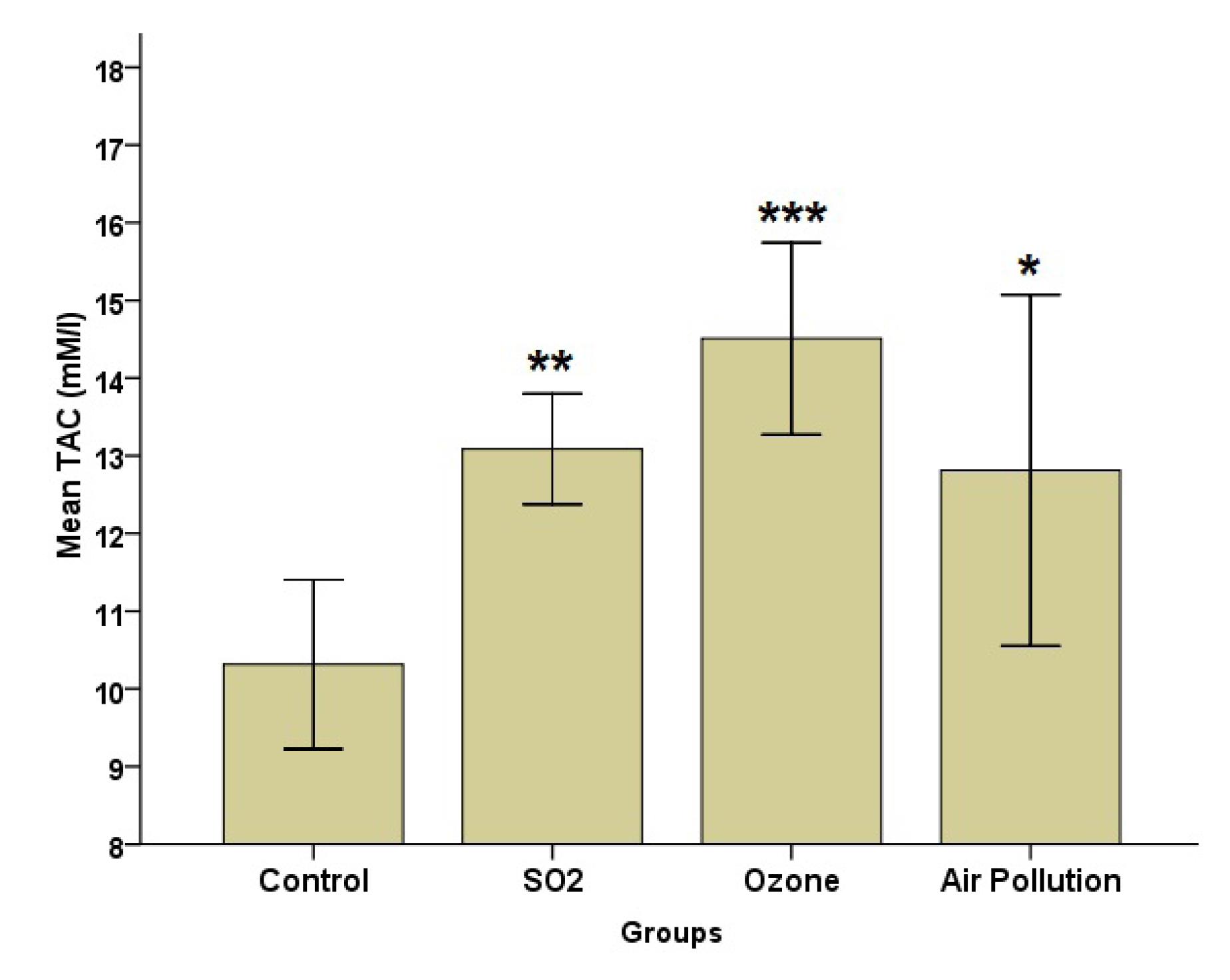
Fig. 1.
Effects of SO2, O3, and Ambient Air Pollution Inhalation on the Blood Serum of TAC (Mean ± SD, n = 8). Note. SO2: Sulfur dioxide; O3: Ozone: SD: Standard deviation; TAC: Total antioxidant capacity. *,**, and *** represent significant differences with the control group (P < 0.05, P < 0.01, and P < 0.001 levels, respectively)
.
Effects of SO2, O3, and Ambient Air Pollution Inhalation on the Blood Serum of TAC (Mean ± SD, n = 8). Note. SO2: Sulfur dioxide; O3: Ozone: SD: Standard deviation; TAC: Total antioxidant capacity. *,**, and *** represent significant differences with the control group (P < 0.05, P < 0.01, and P < 0.001 levels, respectively)
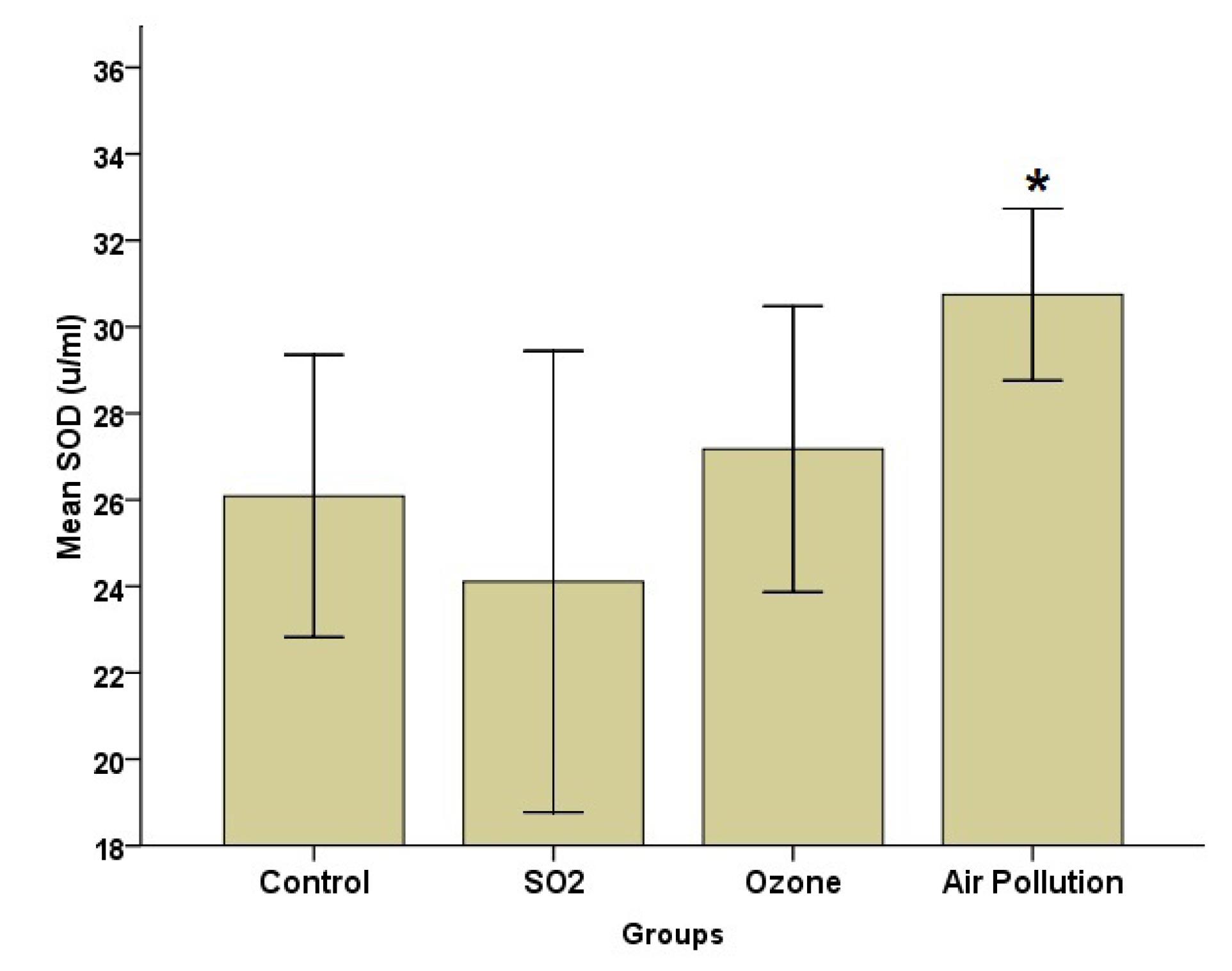
Fig. 2.
Effects of SO2, O3, and AAP Inhalation on the Blood Serum of SOD (Mean ± SD, n = 8). Note. SO2: Sulfur dioxide; O3: Ozone: SD: Standard deviation; AAP: Ambient air pollution; SOD: Superoxide dismutase. * denotes significant differences with the SO2 group (P< 0.05)
.
Effects of SO2, O3, and AAP Inhalation on the Blood Serum of SOD (Mean ± SD, n = 8). Note. SO2: Sulfur dioxide; O3: Ozone: SD: Standard deviation; AAP: Ambient air pollution; SOD: Superoxide dismutase. * denotes significant differences with the SO2 group (P< 0.05)
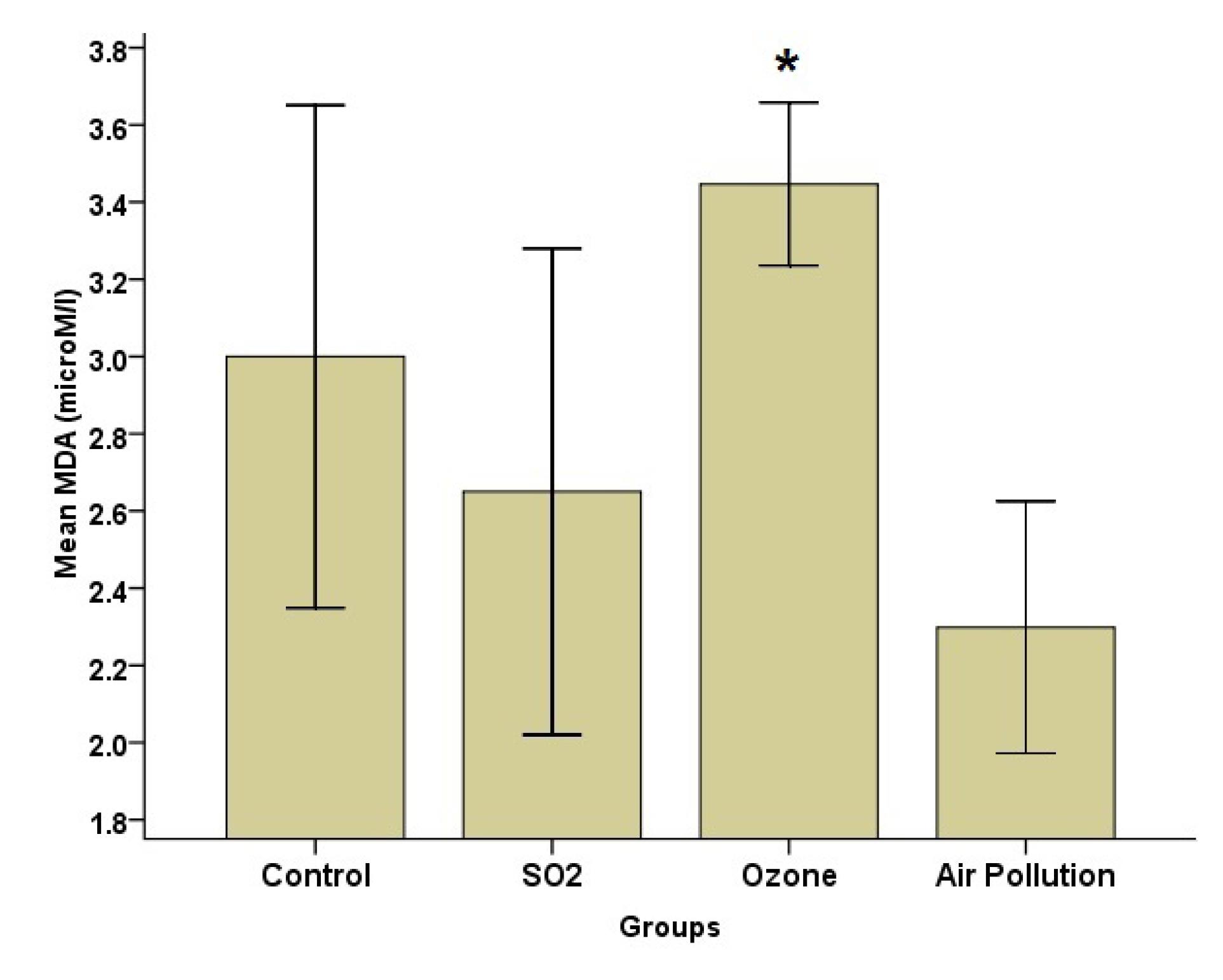
Fig. 3.
Effects of SO2, O3, and Ambient Air Pollution Inhalation on the Blood Serum of MDA (Mean ± SD, n = 8). Note. SO2: Sulfur dioxide; O3: Ozone: SD: Standard deviation; AAP: Ambient air pollution; MDA: Malondialdehyde. * indicates significant differences with the AAP group (P < 0.05)
.
Effects of SO2, O3, and Ambient Air Pollution Inhalation on the Blood Serum of MDA (Mean ± SD, n = 8). Note. SO2: Sulfur dioxide; O3: Ozone: SD: Standard deviation; AAP: Ambient air pollution; MDA: Malondialdehyde. * indicates significant differences with the AAP group (P < 0.05)
According to Fig. 2, the SOD level was the highest in the AAP group, while it was the lowest in the SO2 group. Group analysis, however, revealed that there was a significant difference (P = 0.05) between the AAP and control groups. The MDA data (Fig. 3) indicated that the AAP group had lower MDA levels than the other groups (P = 0.022). This difference, however, only matters when comparing the AAP and O3 groups (P = 0.018). Despite group differences in GPx, the results represented no statistically significant difference (Fig. 4).
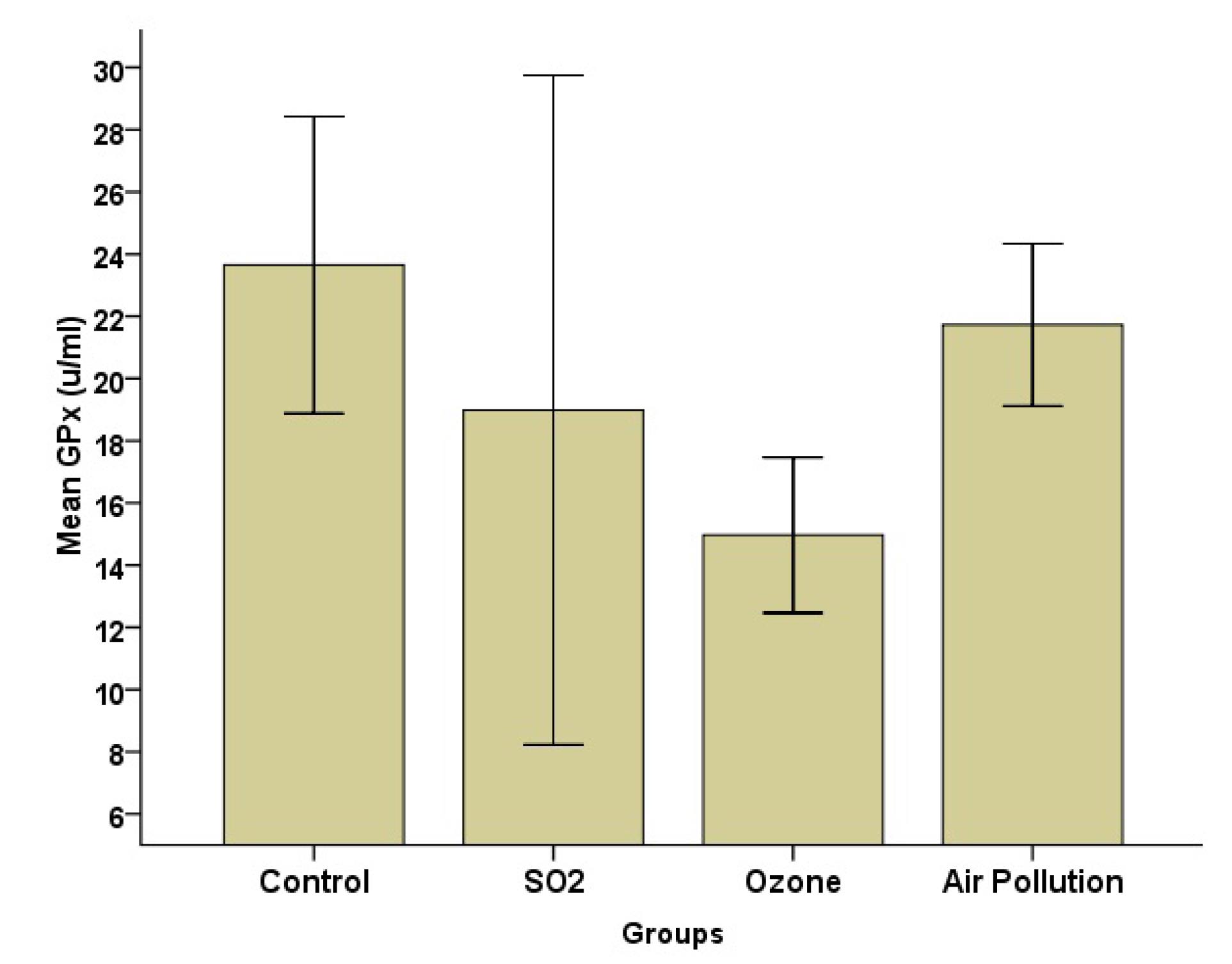
Fig. 4.
Effects of SO2, O3, and Ambient Air Pollution Inhalation on the Blood Serum of GPx (Mean ± SD, n = 8). Note. SO2: Sulfur dioxide; O3: Ozone: SD: Standard deviation; GPx: Glutathione peroxidase
.
Effects of SO2, O3, and Ambient Air Pollution Inhalation on the Blood Serum of GPx (Mean ± SD, n = 8). Note. SO2: Sulfur dioxide; O3: Ozone: SD: Standard deviation; GPx: Glutathione peroxidase
Catalase levels in the SO2 and control groups were comparable, and in the other exposure groups, they were lower than in the SO2 or control groups without statistical significance (Fig. 5).
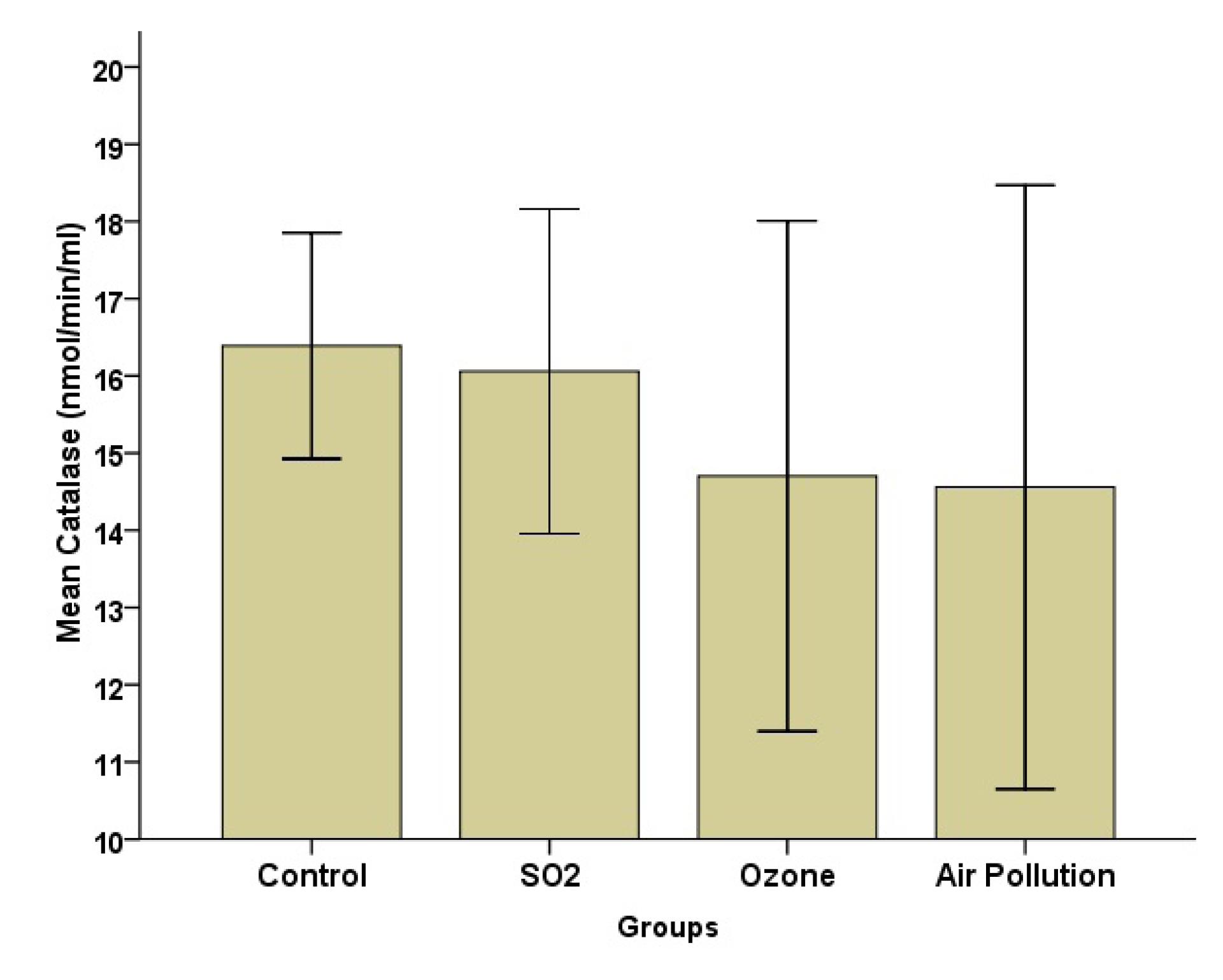
Fig. 5.
Effects of SO2, O3, and Ambient Air Pollution Inhalation on the Blood Serum of Catalase (Mean ± SD, n = 8). Note. SO2: Sulfur dioxide; O3: Ozone: SD: Standard deviation
.
Effects of SO2, O3, and Ambient Air Pollution Inhalation on the Blood Serum of Catalase (Mean ± SD, n = 8). Note. SO2: Sulfur dioxide; O3: Ozone: SD: Standard deviation
3.3. Changes in the Blood Serum of Lipid Profiles After Ambient Air Pollution and Various Gaseous Exposure
The results related to the effects of five weeks of exposure to SO2, O3, and AAP on the blood serum of lipid profiles are provided in Table 1. There were no discernible differences in LDL, triglyceride, or cholesterol levels across the groups. However, those in the O3 group had greater HDL levels. Based on the group analysis, only the difference between the O3 and control groups was significant (P = 0.003).
Table 1.
Changes in the Blood Serum of Lipid Profiles in Different Study Groups (Mean ± SD)
|
Parameters
|
Control
(n=8)
|
SO2
(n=8)
|
O3
(n=8)
|
AAP (n=8)
|
P
Value
(Between Groups)
|
| Triglyceride |
73.20 (25.06) |
84.70 (25.30) |
71.67 (22.95) |
70.73 (28.17) |
0.710 |
| Cholesterol |
69.52 (12.21) |
72.84 (19.66) |
60.20 (11.17) |
70.78 (11.39) |
0.349 |
| HDL |
32.36 (5.30) |
39.13 (5.52) |
47.52 (10.35) |
36.71 (7.75) |
0.005 |
| LDL |
21.30 (1.96) |
21.23 (0.88) |
20.31 (0.97) |
21.85 (1.23) |
0.206 |
Note. SO2: Sulfur dioxide; O3: Ozone: SD: Standard deviation; HDL: High-density lipoprotein cholesterol; LDL: Low-density lipoprotein cholesterol; AAP: Ambient air pollution.
This study examined the association between AAP, SO2, and O3 effects on the blood serum of the lipid profile and OS of male Wistar rats. The mean ambient air SO2, NO2, and O3 levels during the investigation (in the AAP group) were according to the guideline values of the WHO (20 μg/m3, 40 μg/m3, and 100 μg/m3,respectively), but PM2.5 and PM10 concentrations exceeded the guideline values (10 μg/m3 and 20 μg/m3, respectively). The results indicated that blood serum TAC and SOD significantly increased following exposure to AAP. Exposure to air pollution may have stimulated TAC and SOD levels. AAP exposure did not significantly alter the lipid profiles of the groups in our investigation. As in the report of Bernardi et al (26), the present study’s AAP group had higher levels of SOD and TAC than the control group.
After fossil fuels are burned, another air pollutant called SO2 is emitted into the atmosphere. Derivatives of SO2 can enter the bloodstream and affect other body fluids (27). Similar to the pollutants previously described, it may involve oxidative damage to cells, tissues, and organs brought on by sulfur- and oxygen-centered free radicals produced during the oxidation of sulfite (28). Wang et al (29) did not find any linear relationship between exposure to SO2 and the lipid profile of study subjects, which is compatible with our findings. In research by Wu et al (30), long-term exposure to SO2 decreased the level of HDL.
Meng determined the role of SO2 increment in lipid peroxidation and altered intracellular redox status in mice by administering 20 ppm (56 mg/m3) SO2 to the animals for six hours per day for a week. They revealed that SO2 inhalation decreased SOD and GPx compared to the control group (31). In our study, the level of catalase in SO2-exposed rats was similar to that of the control group; in addition, the levels of SOD and GPx were lower than those of the control group, which is consistent with their results. Moreover, in the present research, only the increase in TAC was statistically significant in the SO2 group. This is probably because of the effects of stimuli on oxidative defense enzymes by producing OS.
The elevation of serum TAC after O3 exposure was greater than that of SO2. This is possibly because of the significant elevation in the blood serum of HDL after O3 exposure. HDL has antioxidant properties and may help with TAC elevation. Neutrophilic inflammation occurs after acute exposure to O3, while oxidative pathway activation and, consequently, cell death are the outcomes of chronic exposure (32).
In this study, the blood serum level of MDA significantly increased after O3 exposure. MDA is a byproduct of reactive oxygen species-induced unsaturated fatty acid oxidation, studied as a biomarker of OS. An increment in the MDA level can result from an increase in lipid peroxidation, which can be a possible result of OS induction. There is strong evidence that redox-sensitive signaling pathways mediate the pulmonary inflammatory response after exposure to a contaminant. In addition, a person’s susceptibility to infection appears to be related to their antioxidant defenses (33).
The exact mechanisms of the links between air pollutants and lipid metabolism are unknown. Inhaled pollutants can induce systemic inflammation and OS, which can lead to poor lipid metabolism and oxidation. This is one proposed biological mechanism. Additionally, by decreasing the activity of DNA methyltransferases, air contaminants cause incorrect DNA methylation. Earlier studies examined the relationship between exposure to air pollutants and aberrant DNA methylation, as well as methylation in certain genes related to fat metabolism (8,34). According to a systematic review, there is a high correlation between blood lipids and air pollution in overweight and obese people (8), which is in line with the results of a previously published study (35). Air pollution is thought to be the cause of increased systemic inflammation in these situations (8).
Examining the impact of different air pollution exposures on OS indicators and lipid profiles is one of our study’s strong points. To precisely determine the process underlying the adverse effects that air pollutants cause on organs, it is advised to assess these parameters on several animal model organs.
4. Conclusion
According to our findings, exposure to AAP, SO2, and O3 at the levels used in this investigation may increase overall antioxidant capacity via stimulating blood serum oxidative defense enzymes. Other epidemiological studies are required to evaluate the impacts of other air contaminants on biochemical markers.
Acknowledgments
This research was financially supported by the Drug Applied Research Center of Tabriz University of Medical Sciences, Tabriz, Iran (grant No. 62144).
Authors’ Contribution
Conceptualization: Sorayya Kheirouri, Mohammad Alizadeh.
Data curation: Roya Darbani.
Formal analysis: Roya Darbani.
Funding acquisition: Mehran Mesgari-Abbasi.
Investigation: Mehran Mesgari-Abbasi, Mohammad Alizadeh.
Methodology: Mohammad Alizadeh, Mehran Mesgari-Abbasi.
Project administration: Sorayya Kheirouri, Mehran Mesgari-Abbasi.
Resources: Sorayya Kheirouri, Mehran Mesgari-Abbasi.
Software: Oldouz Rabet.
Supervision: Mehran Mesgari-Abbasi.
Validation: Hossein Mashhadi-Abdolahi.
Laboratory: Oldouz Rabet, Roya Darbani.
Visualization: Asal Golchin.
Writing–original draft: Hossein Mashhadi-Abdolahi.
Writing–review & editing: Asal Golchin.
Competing Interests
None declared.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical Approval
The animal procedures of the study were surveyed, approved, and monitored by the Animal Research Ethics Committee located in Tabriz University of Medical Sciences under the certificate number of IR.TBZMED.REC.1399.083) (2020-06-15).
Funding
This research was financially supported by the Drug Applied Research Center of the Tabriz University of Medical Sciences, Tabriz, Iran (grant no. 62144).
References
- Dhimal M, Neupane T, Lamichhane Dhimal M. Understanding linkages between environmental risk factors and noncommunicable diseases-a review. FASEB Bioadv 2021; 3(5):287-94. doi: 10.1096/fba.2020-00119 [Crossref] [ Google Scholar]
- Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut 2008; 151(2):362-367. doi: 10.1016/j.envpol.2007.06.012 [Crossref] [ Google Scholar]
- Pratiwi YE, Taufik FF, Habibi J, Wibowo A. The impact of particulate matter on the respiratory system. Jurnal Respirasi 2023; 9(3):237-45. doi: 10.20473/jr.v9-I.3.2023.237-245 [Crossref] [ Google Scholar]
- Basith S, Manavalan B, Shin TH, Park CB, Lee WS, Kim J. The impact of fine particulate matter 25 on the cardiovascular system: a review of the invisible killer. Nanomaterials (Basel) 2022; 12(15):2656. doi: 10.3390/nano12152656 [Crossref] [ Google Scholar]
- World Health Organization (WHO). WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 And PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. WHO; 2021.
- Fatmi Z, Mahmood S, Samad Z, Wasay M. Air pollution and non-communicable diseases. J Pak Med Assoc 2020; 70(11):1875-7. [ Google Scholar]
- Meo SA, Suraya F. Effect of environmental air pollution on cardiovascular diseases. Eur Rev Med Pharmacol Sci 2015; 19(24):4890-7. [ Google Scholar]
- Gaio V, Roquette R, Dias CM, Nunes B. Ambient air pollution and lipid profile: systematic review and meta-analysis. Environ Pollut 2019; 254(Pt B):113036. doi: 10.1016/j.envpol.2019.113036 [Crossref] [ Google Scholar]
- Yeatts K, Svendsen E, Creason J, Alexis N, Herbst M, Scott J. Coarse particulate matter (PM25-10) affects heart rate variability, blood lipids, and circulating eosinophils in adults with asthma. Environ Health Perspect 2007; 115(5):709-14. doi: 10.1289/ehp.9499 [Crossref] [ Google Scholar]
- Bind MA, Peters A, Koutrakis P, Coull B, Vokonas P, Schwartz J. Quantile regression analysis of the distributional effects of air pollution on blood pressure, heart rate variability, blood lipids, and biomarkers of inflammation in elderly American men: the normative aging study. Environ Health Perspect 2016; 124(8):1189-98. doi: 10.1289/ehp.1510044 [Crossref] [ Google Scholar]
- Cai Y, Hansell AL, Blangiardo M, Burton PR, de Hoogh K, Doiron D. Long-term exposure to road traffic noise, ambient air pollution, and cardiovascular risk factors in the HUNT and lifelines cohorts. Eur Heart J 2017; 38(29):2290-6. doi: 10.1093/eurheartj/ehx263 [Crossref] [ Google Scholar]
- Yitshak Sade M, Kloog I, Liberty IF, Schwartz J, Novack V. The association between air pollution exposure and glucose and lipids levels. J Clin Endocrinol Metab 2016; 101(6):2460-7. doi: 10.1210/jc.2016-1378 [Crossref] [ Google Scholar]
- Shanley RP, Hayes RB, Cromar KR, Ito K, Gordon T, Ahn J. Particulate air pollution and clinical cardiovascular disease risk factors. Epidemiology 2016; 27(2):291-8. doi: 10.1097/ede.0000000000000426 [Crossref] [ Google Scholar]
- Yang BY, Guo Y, Markevych I, Qian ZM, Bloom MS, Heinrich J. Association of long-term exposure to ambient air pollutants with risk factors for cardiovascular disease in China. JAMA Netw Open 2019; 2(3):e190318. doi: 10.1001/jamanetworkopen.2019.0318 [Crossref] [ Google Scholar]
- Fioravanti S, Cesaroni G, Badaloni C, Michelozzi P, Forastiere F, Porta D. Traffic-related air pollution and childhood obesity in an Italian birth cohort. Environ Res 2018; 160:479-86. doi: 10.1016/j.envres.2017.10.003 [Crossref] [ Google Scholar]
- Tripathi R, Gupta R, Sahu M, Srivastava D, Das A, Ambasta RK. Free radical biology in neurological manifestations: mechanisms to therapeutics interventions. Environ Sci Pollut Res Int 2022; 29(41):62160-207. doi: 10.1007/s11356-021-16693-2 [Crossref] [ Google Scholar]
- Engwa GA, EnNwekegwa FN, Nkeh-Chungag BN. Free radicals, oxidative stress-related diseases and antioxidant supplementation. Altern Ther Health Med 2022; 28(1):114-28. [ Google Scholar]
- Di Meo S, Venditti P. Evolution of the knowledge of free radicals and other oxidants. Oxid Med Cell Longev 2020; 2020:9829176. doi: 10.1155/2020/9829176 [Crossref] [ Google Scholar]
- Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep 2017; 19(11):42. doi: 10.1007/s11883-017-0678-6 [Crossref] [ Google Scholar]
- Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 2011; 301(6):H2181-90. doi: 10.1152/ajpheart.00554.2011 [Crossref] [ Google Scholar]
- Ramond A, Godin-Ribuot D, Ribuot C, Totoson P, Koritchneva I, Cachot S. Oxidative stress mediates cardiac infarction aggravation induced by intermittent hypoxia. Fundam Clin Pharmacol 2013; 27(3):252-61. doi: 10.1111/j.1472-8206.2011.01015.x [Crossref] [ Google Scholar]
- Klaunig JE. Oxidative stress and cancer. Curr Pharm Des 2018; 24(40):4771-8. doi: 10.2174/1381612825666190215121712 [Crossref] [ Google Scholar]
- Pant A, Joshi RC, Sharma S, Pant K. Predictive modeling for forecasting air quality index (AQI) using time series analysis. Avicenna J Environ Health Eng 2023; 10(1):38-43. doi: 10.34172/ajehe.2023.5376 [Crossref] [ Google Scholar]
- Sarvi F, Nadali A, Khodadost M, Kharghani Moghaddam M, Sadeghifar M. Application of Poisson hidden Markov model to predict number of PM25 exceedance days in Tehran during 2016-2017. Avicenna J Environ Health Eng 2017; 4(1):e58031. doi: 10.5812/ajehe.58031 [Crossref] [ Google Scholar]
- Kheirouri S, Shanehbandi D, Khordadmehr M, Alizadeh M, Eskandari Vaezi F, Musapour Sultan Abad R. Effects of sulfur dioxide, ozone, and ambient air pollution on lung histopathology, oxidative-stress biomarkers, and apoptosis-related gene expressions in rats. Exp Lung Res 2022; 48(3):137-48. doi: 10.1080/01902148.2022.2072977 [Crossref] [ Google Scholar]
- Bernardi RB, Zanchi AC, Damaceno-Rodrigues NR, Veras MM, Nascimento Saldiva PH, Tannhauser Barros HM. The impact of chronic exposure to air pollution over oxidative stress parameters and brain histology. Environ Sci Pollut Res Int 2021; 28(34):47407-17. doi: 10.1007/s11356-021-14023-0 [Crossref] [ Google Scholar]
- Koenig JQ. Health Effects of Ambient Air Pollution: How Safe is the Air We Breathe? Springer Science & Business Media; 2000.
- Ubuka T, Yuasa S, Ohta J, Masuoka N, Yao K, Kinuta M. Formation of sulfate from L-cysteine in rat liver mitochondria. Acta Med Okayama 1990; 44(2):55-64. doi: 10.18926/amo/30442 [Crossref] [ Google Scholar]
- Wang M, Zheng S, Nie Y, Weng J, Cheng N, Hu X. Association between short-term exposure to air pollution and dyslipidemias among type 2 diabetic patients in northwest China: a population-based study. Int J Environ Res Public Health 2018; 15(4):631. doi: 10.3390/ijerph15040631 [Crossref] [ Google Scholar]
- Wu XM, Basu R, Malig B, Broadwin R, Ebisu K, Gold EB. Association between gaseous air pollutants and inflammatory, hemostatic and lipid markers in a cohort of midlife women. Environ Int 2017; 107:131-9. doi: 10.1016/j.envint.2017.07.004 [Crossref] [ Google Scholar]
- Meng Z. Oxidative damage of sulfur dioxide on various organs of mice: sulfur dioxide is a systemic oxidative damage agent. Inhal Toxicol 2003; 15(2):181-95. doi: 10.1080/08958370304476 [Crossref] [ Google Scholar]
- Wiegman CH, Li F, Ryffel B, Togbe D, Chung KF. Oxidative stress in ozone-induced chronic lung inflammation and emphysema: a facet of chronic obstructive pulmonary disease. Front Immunol 2020; 11:1957. doi: 10.3389/fimmu.2020.01957 [Crossref] [ Google Scholar]
- Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med 2003; 60(8):612-6. doi: 10.1136/oem.60.8.612 [Crossref] [ Google Scholar]
- Chen Z, Salam MT, Toledo-Corral C, Watanabe RM, Xiang AH, Buchanan TA. Ambient air pollutants have adverse effects on insulin and glucose homeostasis in Mexican Americans. Diabetes Care 2016; 39(4):547-54. doi: 10.2337/dc15-1795 [Crossref] [ Google Scholar]
- Sørensen M, Hjortebjerg D, Eriksen KT, Ketzel M, Tjønneland A, Overvad K. Exposure to long-term air pollution and road traffic noise in relation to cholesterol: a cross-sectional study. Environ Int 2015; 85:238-43. doi: 10.1016/j.envint.2015.09.021 [Crossref] [ Google Scholar]