Avicenna J Environ Health Eng. 9(2):93-99.
doi: 10.34172/ajehe.2022.5304
Original Article
Removal Efficiency of Electrochemical Process by Iron Oxide Nanoparticles Impregnated on Rod Carbon for Arsenite Anions in a High Ionic Strength Solution
Mahdiah Soltani 1  , Ehsan Abouee Mehrizi 1, Mohammad Taghi Ghaneian 1, Mohammad Hossein Salmani 2, *
, Ehsan Abouee Mehrizi 1, Mohammad Taghi Ghaneian 1, Mohammad Hossein Salmani 2, * 
Author information:
1Environmental Science and Technology Research Center, Department of Environmental Health Engineering, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
2Research Center for Food Hygiene and Safety, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Abstract
The entry of heavy metals (e.g., arsenite anions) into water sources due to industrial and mining activities is considered a serious problem for the environment. Therefore, arsenite removal from polluted water has attracted the attention of researchers due to its toxic effects on human health. In the present study, the efficiency of the electrochemical (EC) purification of arsenite anions in a high ionic strength solution was investigated using a carbon electrode impregnated with iron oxide nanoparticles (NPs). These NPs were synthesized by the co-precipitation method and immediately impregnated on the carbon rod. Experiments were performed by two electrodes (carbon and carbon impregnated with NP electrode) in a 50 mL EC reactor. The effect of different parameters such as electrical current (0.1, 0.3, 0.5, 0.7, & 0.9 A), EC time (2, 5, 10, 15, and 20 minutes), pH (3, 5, 7, 9, & 11), and the initial concentrations of arsenite anion (2, 5, 10, 15 and 20 mg/L) was investigated on the removal efficiency to achieve the highest removal of arsenite anions. Arsenite anions (10 mg/L) were completely removed from the aqueous solution with an ionic strength of 0.141 M at an electrical current of 0.7 A, a pH of 8, and an EC time of 20 minutes. In addition, pH was the most effective parameter in removing arsenite anions from aqueous solution in the EC method. According to the results, EC treatment using an electrode impregnated with iron oxide NPs is highly efficient in removing arsenite anions from the contaminated water.
Keywords: Arsenite anions, Electrochemical process, Iron oxide nanoparticles, High ionic strength, Removal efficiency,
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Soltani M, Abouee Mehrizi E, Ghaneian MT, Salmani MH. Removal efficiency of electrochemical process by iron oxide nanoparticles impregnated on rod carbon for arsenite anions in a high ionic strength solution. Avicenna J Environ Health Eng. 2022; 9(2):93-99. doi:10.34172/ajehe.2022.5304
1. Introduction
At present, access to healthy water sources is difficult due to the industrialization of societies, rapid population growth, and reduced rainfall. The most important factor in the pollution of water sources is the entry of heavy and toxic elements into the environment caused by industrial and mining activities (1). Heavy metals in industrial effluents after discharge into the environment are serious threats to surface and groundwater sources. Arsenic usually exists in the form of arsenate (AsO43-) or arsenite (AsO33-) in water sources, where the arsenite anion is about 60 times more toxic and dangerous than the arsenate anion (2). Arsenic enters surface and groundwater sources naturally (due to weathering of minerals and rock and soil erosion) or due to human activities such as mining and the like (3,4). The presence of arsenic in the water of some regions of the world can be considered a chronic problem, among which we can mention East and Southeast Asian countries such as Bangladesh, Thailand, and Malaysia, as well as Western America, Chile, and Argentina (5). In Iran, in areas such as Sistan and Baluchistan, Kurdistan, and East Azerbaijan, the amount of arsenic in water sources is higher than the standard limit (6,7). Contamination of drinking water with heavy metals, especially arsenite anions, is a problem that has endangered the health of many people (8). Depending on the arsenic concentration and contact time, arsenic can lead to acute (vomiting and tingling of hands and feet) and chronic (cardiovascular diseases, immune, nervous, and reproductive system disorders) poisoning (9). Due to the harmful effects of arsenic on humans, the Environmental Protection Agency has decreased the maximum concentration of arsenic in drinking water from 50 to 10 μg/L (10,11).
Different methods such as coagulation and flocculation, ion exchange, membrane processes, chemical precipitation, and surface adsorption have been used to remove arsenic anions from aqueous solutions (9,12,13). Contrary to their widespread use, the above-mentioned methods have many problems such as high maintenance costs, excessive consumption of chemicals, and sludge management (14,15). In addition, most arsenic removal technologies are effective in removing arsenate and have a low capacity in removing arsenite because arsenite form is not normally hydrolyzed up to a pH rate of 9, and its solubility is low. In addition, arsenate has a greater affinity for inorganic compounds or has adsorption on solid surfaces such as iron oxide. Therefore, to completely remove arsenic compounds, first, the oxidation step must be performed to convert arsenite into arsenate.
In recent years, electrochemical (EC) processes as an advanced and efficient process for the treatment of industrial wastewater have attracted the attention of many chemical and environmental health researchers (16,17). The base of the EC process is to apply direct current to the electrodes of an EC cell and adsorb ions along with oxidation and reduction reactions on the surface of the electrodes. The most important advantages of this method are operation at ambient temperature and pressure, low use of chemicals and thus less sludge production, the ability to adapt to changes in the composition and initial flow rate, and the ability to remove a wide range of pollutants (18).
Iron oxide has been extended to remove pollutants from water and wastewater and has an important role in a variety of industrial applications. Several kinds of iron oxide have been studied for arsenic anion adsorption from contaminated solutions. Some types of iron oxide are used as the adsorbents of arsenic anions in the solution, including goethite, magnetite, hematite, siderite, limonite, and ferrihydrite. The most widespread types of iron oxide are magnetite (Fe3O4) and ferrihydrite (FeOOH). The results of the study by Thirunavukkarasu et al indicated that arsenite is more strongly adsorbed on ferrihydrite than arsenate in the pH range of 3-11 at a high initial arsenic concentration (19).
Thus, this study was conducted to determine EC efficiency for the treatment of arsenite anions in a high ionic strength solution using a rod carbon electrode impregnated with iron oxide NPs. The influences of electrical current, EC time, solution pH, and initial arsenite concentration were studied in batch experiments.
2. Materials and Methods
This experimental study was conducted on a semi-scale in the Chemistry Laboratory of Yazd Health Faculty. First, a rod carbon electrode was modified with iron oxide by the co-precipitation method, and an EC reactor was designed to investigate the arsenite removal. After variable determination and according to study objectives, EC examinations were performed to obtain high removal efficiency by the EC reactor. The steps of the study are specified as follows:
2.1. Synthesis of iron oxide NPs
Iron oxide NPs were synthesized by the co-precipitation method for impregnation on the rod carbon electrode. For this purpose, 0.1 M solution of iron (III) by dissolving 5.35 g of FeCl3, and 0.1 M solution of iron (II) by dissolving 8.10 g of FeCl2.4H2O were separately prepared in 500 mL of water. Then, its temperature was raised to 80°C, and two solutions were mixed together. Finally, 0.1 M of ammonium hydroxide solution was slowly added dropwise for 20 minutes until the pH of the solution reached about 9. The obtained solution was placed in an ultrasonic bath for 10 minutes (20,21).
2.2. Impregnation of NPs on Rod Carbon
The used electrode in this study was a rod carbon of the applied batteries with a length of 4.5 cm and a diameter of 0.5 cm. The rod carbon was washed with soapy water and cleaned with a plastic brush. Then, its surface was smoothed and polished with the 3% HNO3 solution. The polished rod carbon was immersed in the freshly synthesized iron oxide NP solution for 2 hours on the shaker. The formation of iron oxide NPs on the rod carbon was controlled by the scanning electron microscope (SEM). The rod carbon impregnated with iron oxide NPs was washed 3 times with distilled water and ethanol (70:30) and dried at laboratory temperature, and this electrode was kept in a desiccator for further EC experiments. The schematic of the iron oxide NP synthesis process and impregnation on rod carbon is illustrated in Fig. 1.
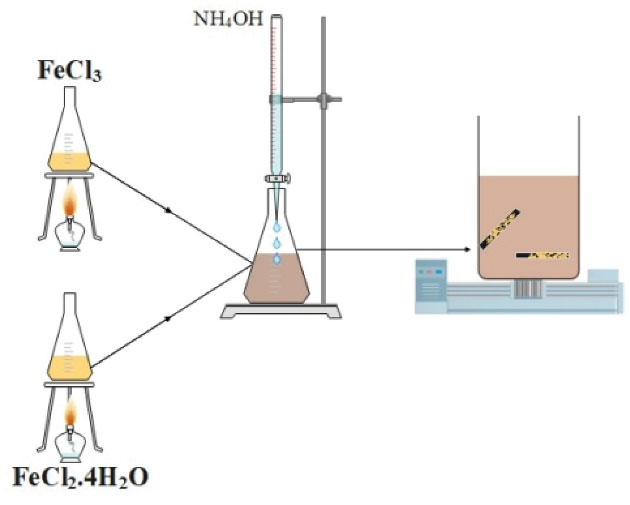
Fig. 1.
The Schematic of Synthesis and Impregnation of Nanoparticles on Rod Carbon
.
The Schematic of Synthesis and Impregnation of Nanoparticles on Rod Carbon
2.3. EC Process
To perform the experiments, a cubic reactor 2 × 2 × 5 cm with a volume of 50 mL was employed with two carbon rods (one without NPs and the other impregnated with iron oxide NPs) at a distance of 3 cm, connected to the power source as cathode and anode. Fig. 2 depicts the schematic of the reactor:
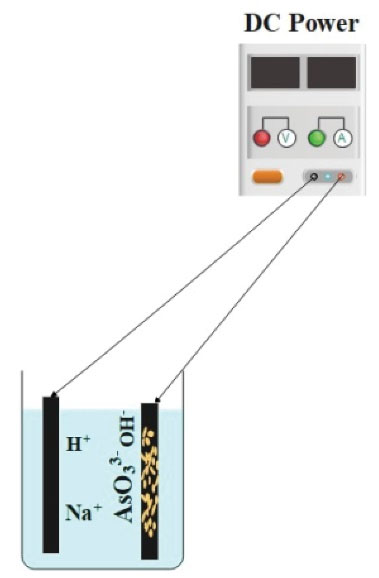
Fig. 2.
The Schematic of the Electrochemical Reactor
.
The Schematic of the Electrochemical Reactor
To prepare the desired solutions of arsenite in the laboratory, first, the mother solution of arsenite was prepared from sodium arsenite (Na2HASO3). The desired concentrations were daily prepared by diluting the mother solution with distilled water (6,22). All experiments were performed at a temperature of 20 ± 2°C. To increase the ionic strength and electrical conductivity of the solution, 2 mL of Na2SO4 electrolyte with a concentration of 10 g/L was added to the reactor. Next, 0.1 M of the H2SO4 or M NaOH solution was also used to adjust the pH. The effect of different parameters, including electrical current (0.3, 0.5, 0.7, & 0.9 A), EC time (2, 5, 10, 15, 20 minutes), pH (3, 5, 7, 9, & 11), and the initial concentration of arsenic (2, 5, 10, 15, 20 mg/L), on the removal efficiency of arsenite anions in high ionic strength solutions (0.141 M) was determined as a factor at any time in a batch reactor. At the end of each test, the electrodes were washed with distilled water, placed in a 1% hydrochloric acid solution for 20 minutes, then rinsed well, and dried. The experiments were repeated twice, and the average results were reported at the end. To measure the residual arsenic concentration in the water after the EC process, the solution was centrifuged and measured using an atomic absorption spectrometer at a wavelength of 193.7 nm by an acetylene-nitrous oxide flame (23).
2.4. Data Analysis
The removal efficiency of arsenic in the solution in each step was calculated by Eq. (1) as follows (24):
The adsorbed mass in the EC process was estimated by Faraday Eq. (2) as (25):
The efficiency of the EC cell was obtained by Eq. (3):
The energy consumption of the EC process (kWh/kg analyte) was computed by Eq. (4) as follows (26):
(4)
where C0 and C are the initial and residual concentrations (arsenite anions) in the solution (mg/L), and I is the applied current (A). In addition, t, E, and VR represent EC time (s), the equivalent of pollutant (arsenite anions), and the reactor volume (L), respectively. Further, mcal and V denote the calculated removal mass active specie (arsenite anions) and the applied voltage (V), respectively.
3. Results and Discussion
The morphology of the electrode surface was considered by SEM (Fig. 3). The SEM image illustrated that the whole surface of the rod carbon electrode had been coated with iron oxide NPs.

Fig. 3.
The SEM of the Surface of a Rod Carbon Electrode Impregnated With Iron Oxide. Note. SEM: Scanning Electron Microscope
.
The SEM of the Surface of a Rod Carbon Electrode Impregnated With Iron Oxide. Note. SEM: Scanning Electron Microscope
3.1. The Effect of Arsenite Anion Concentration
To determine the effect of arsenite anion concentration, experiments were performed at an electrical current of 0.5 A, initial pH solution, time interval of 10 minutes, and different concentrations of 2, 5, 10, 15, and 20 mg/L. Based on the obtained results (Fig. 4), first, the removal efficiency increased up to a concentration of 10 mg/L (76.6%). By increasing the initial concentration of arsenite anions from 10 to 20 mg/L, the removal efficiency decreased from 76% to 49%.
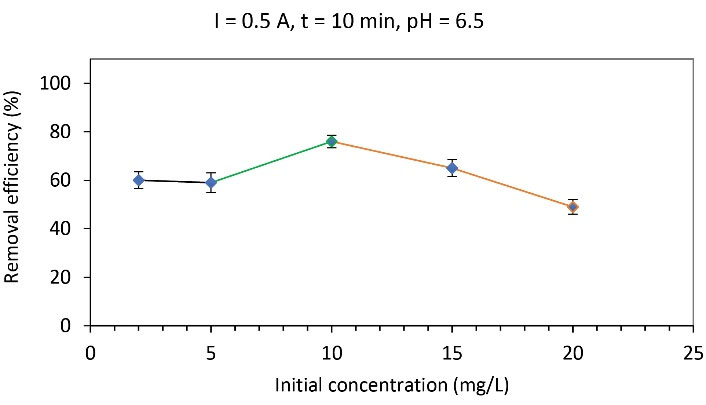
Fig. 4.
The Effect of the Initial Arsenite Concentration on Removal Efficiency
.
The Effect of the Initial Arsenite Concentration on Removal Efficiency
As shown in Fig. 4, the maximum removal efficiency is at the concentration of 10 mg/L of arsenite anions. It is clear that in the EC process, the removal efficiency decreases under the constant conditions of electrical current and EC time when increasing the initial concentration of the substance. In a constant electrical current and voltage, the speed of movement of charged particles (anions and cations) toward the electrodes is constant and the EC process can adsorb and remove a certain amount of ions in a constant time. Therefore, if the initial concentration of the ionic substance in the solution increases, the amount of anions and cations in the EC reactor increases and the time is not enough to remove excess ions; because of the increase of the initial concentration of the substance, not all ions participate in the EC process, and as a result, the efficiency of substance removal represents a reduction (27). Hence, in the present study, it was also observed that removal efficiency decreased with an increase in the concentration of arsenite anions. In the study conducted by Arqiani et al, removal efficiency decreased with increasing the aniline concentration (28). for the removal of phosphate during the EC process using iron and aluminum electrodes, İrdemez et al found that removal efficiency decreased with an increase in the initial concentration of phosphate (29). Thus, the effective concentration to remove arsenite anions in these conditions was 10 mg/L.
3.2. The Effect of pH
According to the initial arsenite concentration determined in the previous step, to explore the effect of pH on the removal efficiency of arsenite anions, experiments were performed with a concentration of 10 mg/L, an electrical current of 0.5 A, and a time interval of 10 minutes at different pH rates of 3, 5, 7, 9, and 11 (Fig. 5). Based on the results, the removal efficiency was low at highly acidic and basic pH rates, and the highest removal efficiency was obtained at a pH rate of 8.
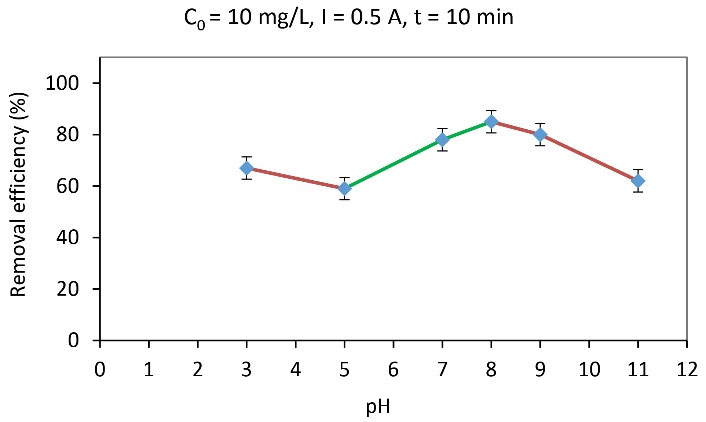
Fig. 5.
The Effect of pH on Removal Efficiency
.
The Effect of pH on Removal Efficiency
According to data in Fig. 5, the highest removal efficiency was 85% (pH = 8). Moreover, a relative increase was observed in removal efficiency at the pH range of 5-8. The pH of the solution significantly affects the speciation of arsenic in the solution, thus the hydrolyzed arsenite species is a function of pH. Arsenite is in the form of neutral H3AsO3 at pH < 9; when pH increases to 9-12, 12-13, and > 13, these particles change from neutral to mono-anionic form H2AsO3-, di-anionic form HAsO32-, and AsO33-, respectively. Therefore, the capacity of the chemical adsorption of arsenic on iron oxide surfaces varies with a change in solution pH (2). In this study, as the pH increased, the effective anions in the solution increased, leading to an increase in the removal percentage of arsenite anions. In addition, at pH > 6, the electrode impregnated with iron oxide NPs has a surface charge and can adsorb with the formation of a complex by arsenite anions. Previous studies also demonstrated that As(III) adsorption on these materials had a maximum pH value of around 8.5, and pH had a direct effect on removal efficiency due to its effect on the electrode surface and arsenic form (30). Accordingly, the arsenite removal from polluted water increased with increasing pH; other researchers attributed it to the difference between HAsO32- dissociation constants (31,32).
3.3. The Effect of Electrochemical Time
To investigate the effect of the EC time on the removal efficiency of arsenite anions, experiments were conducted at a concentration of 10 mg/L, electrical current of 0.5 A, a pH of 8, and time intervals of 2, 5, 10, 15, and 20 minutes. At constant electrical current, the removal efficiency increased with increasing time so that it reached 89% at 20 minutes (Fig. 6).
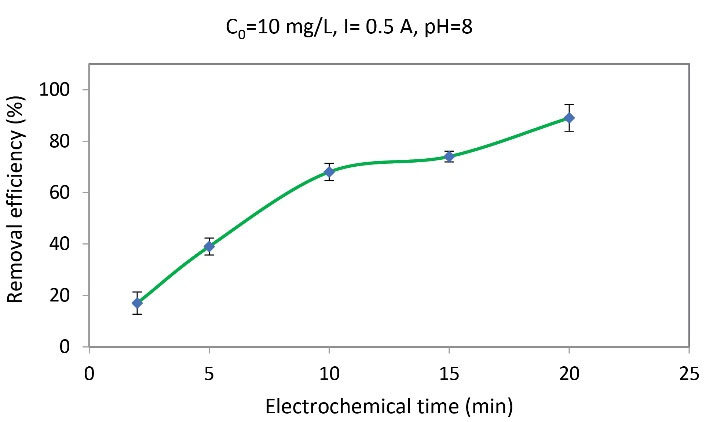
Fig. 6.
The Effect of Electrochemical Time on Removal Efficiency
.
The Effect of Electrochemical Time on Removal Efficiency
Based on Fig. 6, the removal efficiency increased with the increase of the EC time. In the EC process, with increasing time, further electric charge passes through the solution, causing the transfer of more charged particles from the solution to the surface of the electrode while increasing removal efficiency. In the current study, the appropriate reaction time for removing arsenite anions was 20 minutes. The results obtained from the removal of azo dye by the EC process also indicated that by increasing the reaction time from 2 to 14 minutes, the removal efficiency increased to 98% (33). Likewise, Arqiani et al reported that the removal efficiency of aniline increased with an increase in the reaction time (28).
3.4. The Effect of Electrical Current
To determine the effect of the electrical current on the removal efficiency of arsenite anions, the experiments were performed at currents of 0.1, 0.2, 0.3, 0.5, and 0.9 A at the initial concentration of arsenite anions, pH, and time constant (Fig. 7). According to the findings, the maximum removal efficiency (99%) was obtained at an electrical current of 0.7 A.
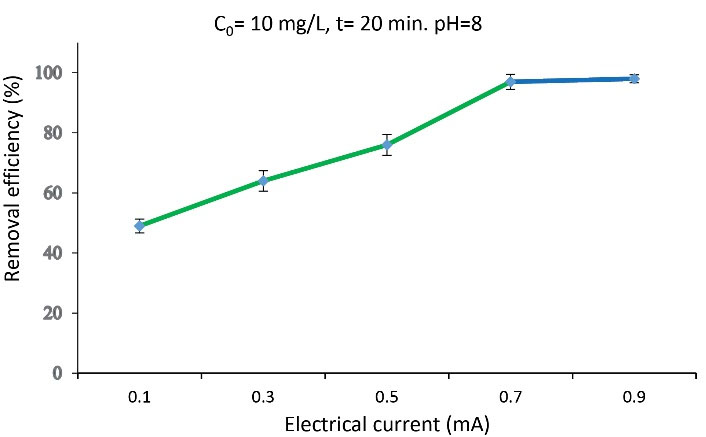
Fig. 7.
The Effect of the Electrical Current on Removal Efficiency
.
The Effect of the Electrical Current on Removal Efficiency
Fig. 7 shows that the removal efficiency of arsenite anions increases with increasing electrical current. Thus, the adsorption of arsenite anions in the anode was significant in all studied currents. Considering the increase in the removal efficiency with increasing in electrical current in the EC process, the transfer rate and the number of charged particles from the solution to the surface of electrodes, which are responsible for pollutant removal, increased with an increase in the electrical current and could increase in the adsorption of arsenite anions on the electrode impregnated with NPs.
3.5. EC Mechanism
The adsorption mechanism of arsenic on iron oxide has been considered by several researchers on the samples of arsenic anion removal by iron oxide using ultraviolet photoelectron spectroscopy and infrared spectroscopy techniques and represented that arsenic anions form monodentate and binuclear bidentate complexes with hydroxyl groups on the iron oxide surface (12-15). Arsenic anions replace one or two OH groups on iron oxide surfaces to form Fe-O-As(OH)-O and Fe-O-AsO(OH)-O-Fe mono or dinuclear bridge complex (34).
The complex form is the most abundant species on iron oxide surfaces and is chemically stable. Therefore, the proposed mechanism involves two steps, including the formation of a monodentate surface complex with a high adsorption rate as covalent bonds between the adsorbed ion and the functional group (-OH) of the surface (35). The anodic oxidation reaction of the arsenite ion and its conversion to an arsenate ion leads to the formation of an internal bidentate complex and increases the affinity of this ion on the surface of iron oxide.
Based on the above discussion, the mechanism of the removal of As(III) by iron oxide surface is adsorption along with the oxidation approach. The whole process can be summarized by reaction equations (5-7).
Anode
(5)
(6)
Cathode
(7)
where (C-O-Fe-O-) represents the adsorption site on the iron oxide adsorbent surface. In addition, C—O-Fe-O-As(O2H) and C—O-Fe-O-AsO3H2 denote arsenic anions (As(III) and As(V)) adsorption on the surface, respectively. This mechanism of adsorption and oxidation can well explain the higher and faster adsorption of As(III) by the EC process.
3.6. Consumed Energy and Adsorbed Mass
Electrical energy consumption is a beneficial factor in the EC process. In such processes, energy consumption is related by several important parameters, including average applied current and voltage, EC time, and mass value reacted in the reactor. Using Eq. (4), in optimum conditions (electrical current of 0.7 A, time interval of 20 minutes, and constant voltage of 11 V), the energy consumption of the unit removal of arsenite ions was 52.3 kWh/kg. Based on electrical energy consumption at optimum conditions, the use of an EC process is a suitable process in the removal of arsenite contaminant from an aqueous solution.
Faraday’s law predicts the mass of a species that can be precipitated, reduced, or oxidized by the amount of electricity that passes through the solution (36). The adsorbed mass on the anode was 35.7 mg, which is calculated by Faraday’s equation (2). The mass of arsenite anions in these deposits is lower than the calculated theoretical values. Using Eq. (3), it was possible to obtain the electric efficiency of the EC process for arsenite removal. The electrical efficiency of the EC process was 75.6%, which is a good practical approach for arsenite anion removal. The obtained result in the present study determined that EC treatment with an electrode impregnated with iron oxide NPs had an acceptable performance for removing arsenite anions.
4. Conclusion
It was found that arsenic anions can be efficiently and rapidly removed from the aqueous solution in the modified EC process. The arsenite anions migrated to the anode by the electrical positive potential, followed by the adsorption and oxidation by the iron oxide on the rod carbon electrode of the anode. The capability of the EC process was closely related to the current, pH, and initial arsenite concentration of the solution. The results indicated that EC efficiency increased with increasing the electrical current, EC time, and proper pH. Under optimal conditions, with a current of 0.7 A, a time of 20 minutes, pH 8, and an initial concentration of arsenite anions of 10 mg/L, 99% of arsenite anions could be removed from the solution. The EC process was used for the removal of arsenite anions to decrease the toxicity of arsenic. Meanwhile, with low energy consumption and eco-friendly conditions, the modified carbon electrode develops the EC process as a promising technology to remove arsenite anions.
Acknowledgments
This research was conducted with the support of the Faculty of Research of Shahid Sadoughi University as an MSc thesis. The authors would like to thank all those who contributed to this research.
Competing Interests
The authors declare that they have no conflict of interests.
References
- Rohani Moghadam M, Talebizadeh Rafsanjani A, Salehi L. Optimization of electrochemical decolorization of Acid Blue 25 by central composite design and desirability function. Appl Chem 2018; 13(46):267-80. doi: 10.22075/chem.2017.2784 [Crossref] [ Google Scholar]
- Gallegos-Garcia M, Ramírez-Muñiz K, Song S. Arsenic removal from water by adsorption using iron oxide minerals as adsorbents: a review. Miner Process Extr Metall 2012; 33(5):301-15. doi: 10.1080/08827508.2011.584219 [Crossref] [ Google Scholar]
- Puente-Urbina A, Montero-Campos V. Porous materials modified with Fe3O4 nanoparticles for arsenic removal in drinking water. Water Air Soil Pollut 2017; 228(9):374. doi: 10.1007/s11270-017-3513-3 [Crossref] [ Google Scholar]
- Tawabini BS, Al-Khaldi SF, Khaled MM, Atieh MA. Removal of arsenic from water by iron oxide nanoparticles impregnated on carbon nanotubes. J Environ Sci Health A Tox Hazard Subst Environ Eng 2011; 46(3):215-23. doi: 10.1080/10934529.2011.535389 [Crossref] [ Google Scholar]
- De D, Mandal SM, Bhattacharya J, Ram S, Roy SK. Iron oxide nanoparticle-assisted arsenic removal from aqueous system. J Environ Sci Health A Tox Hazard Subst Environ Eng 2009; 44(2):155-62. doi: 10.1080/10934520802539756 [Crossref] [ Google Scholar]
- Ghanizadeh G, Safavi SN, Akbari H, Hazrati S. Elimination of arsenic(III) from urban drinking water by electrocoagulation process. J Mil Med 2016;18(2):197-206. [Persian].
- Mosaferi M, Shakerkhatibi M, Dastgiri S, Asghari Jafar-Abadi M, Khataee A, Sheykholeslami S. Natural arsenic pollution and hydrochemistry of drinking water of an urban part of Iran. Avicenna J Environ Health Eng 2014; 1(1):7-16. doi: 10.5812/ajehe.164 [Crossref] [ Google Scholar]
- Feng L, Cao M, Ma X, Zhu Y, Hu C. Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. J Hazard Mater 2012; 217-218:439-46. doi: 10.1016/j.jhazmat.2012.03.073 [Crossref] [ Google Scholar]
- Rahmani Boldaji M, Nabizadeh R, Dehghani MH, Nadafi K, Mahvi AH. Evaluating the performance of iron nanoparticle resin in removing arsenate from water. J Environ Sci Health A Tox Hazard Subst Environ Eng 2010; 45(8):946-50. doi: 10.1080/10934521003772337 [Crossref] [ Google Scholar]
- Chiavola A, Amato ED, Stoller M, Chianese A, Boni MR. Application of iron based nanoparticles as adsorbents for arsenic removal from water. Chem Eng Trans 2016; 47:325-30. doi: 10.3303/cet1647055 [Crossref] [ Google Scholar]
- Luther S, Borgfeld N, Kim J, Parsons JG. Removal of arsenic from aqueous solution: a study of the effects of pH and interfering ions using iron oxide nanomaterials. Microchem J 2012; 101:30-6. doi: 10.1016/j.microc.2011.10.001 [Crossref] [ Google Scholar]
- Chen M, Chen Z, Wu P, Chen JP. Simultaneous oxidation and removal of arsenite by Fe(III)/CaO(2) Fenton-like technology. Water Res 2021; 201:117312. doi: 10.1016/j.watres.2021.117312 [Crossref] [ Google Scholar]
- Laskaridis A, Sarakatsianos I, Tzollas N, Katsoyiannis IA. Simultaneous removal of arsenate and chromate from ground-and surface-waters by iron-based redox assisted coagulation. Sustainability 2020; 12(13):5394. doi: 10.3390/su12135394 [Crossref] [ Google Scholar]
- Koohpayehzadeh H, Torabian A, Nabi Bidhendi G, Habashi N. Nanoparticle zere-valent iron affect on As(V) removal from drinking water. J Water Wastewater 2012;23(3):60-7. [Persian].
- Salmani MH, Abedi M, Mozaffari SA, Mahvi AH, Sheibani A, Jalili M. Simultaneous reduction and adsorption of arsenite anions by green synthesis of iron nanoparticles using pomegranate peel extract. J Environ Health Sci Eng 2021; 19(1):603-12. doi: 10.1007/s40201-021-00631-y [Crossref] [ Google Scholar]
- Doumbi RT, Noumi GB, Domga D. Electrochemical degradation of synthetic textile wastewater by C/MnO2 electrode assessed by surface response methodology. Avicenna J Environ Health Eng 2021; 8(2):116-25. doi: 10.34172/ajehe.2021.15 [Crossref] [ Google Scholar]
- Ehsani H, Mehrdadi N, Asadollahfardi G, Nabi Bidhendi G, Azarian G. A new combined electrocoagulation-electroflotation process for pretreatment of synthetic and real Moquette-manufacturing industry wastewater: optimization of operating conditions. J Environ Chem Eng 2020; 8(5):104263. doi: 10.1016/j.jece.2020.104263 [Crossref] [ Google Scholar]
- Fatehi MH, Shayegan J, Zabihi M. A review of methods for removing heavy metal from aqueous media. Iran J Ecohydrol 2018; 5(3):855-74. doi: 10.22059/ije.2018.249854.804 [Crossref] [ Google Scholar]
- Thirunavukkarasu OS, Viraraghavan T, Subramanian KS. Arsenic removal from drinking water using iron oxide-coated sand. Water Air Soil Pollut 2003; 142(1):95-111. doi: 10.1023/a:1022073721853 [Crossref] [ Google Scholar]
- Yegane Badi M, Azari A, Esrafili A, Ahmadi E, Gholami M. Performance evaluation of magnetized multiwall carbon nanotubes by iron oxide nanoparticles in removing fluoride from aqueous solution. J Mazandaran Univ Med Sci 2015;25(124):128-42. [Persian].
- Ehrampoush MH, Miria M, Salmani MH, Mahvi AH. Cadmium removal from aqueous solution by green synthesis iron oxide nanoparticles with tangerine peel extract. J Environ Health Sci Eng 2015; 13:84. doi: 10.1186/s40201-015-0237-4 [Crossref] [ Google Scholar]
- Bensadok K, El Hanafi N, Lapicque F. Electrochemical treatment of dairy effluent using combined Al and Ti/Pt electrodes system. Desalination 2011; 280(1-3):244-51. doi: 10.1016/j.desal.2011.07.006 [Crossref] [ Google Scholar]
- Farzana Akter K, Chen Z, Smith L, Davey D, Naidu R. Speciation of arsenic in ground water samples: a comparative study of CE-UV, HG-AAS and LC-ICP-MS. Talanta 2005; 68(2):406-15. doi: 10.1016/j.talanta.2005.09.011 [Crossref] [ Google Scholar]
- Hoseinzadeh E, Rezaee A. Electrochemical degradation of RB19 dye using low-frequency alternating current: effect of a square wave. RSC Adv 2015; 5(117):96918-26. doi: 10.1039/c5ra19686h [Crossref] [ Google Scholar]
- Muddemann T, Haupt D, Sievers M, Kunz U. Electrochemical reactors for wastewater treatment. ChemBioEng Rev 2019; 6(5):142-56. doi: 10.1002/cben.201900021 [Crossref] [ Google Scholar]
- Leili M, Shirmohammadi Khorram N, Godini K, Azarian G, Moussavi R, Peykhoshian A. Application of central composite design (CCD) for optimization of cephalexin antibiotic removal using electro-oxidation process. J Mol Liq 2020; 313:113556. doi: 10.1016/j.molliq.2020.113556 [Crossref] [ Google Scholar]
- Panizza M, Cerisola G. Influence of anode material on the electrochemical oxidation of 2-naphthol: part 2 Bulk electrolysis experiments. Electrochim Acta 2004; 49(19):3221-6. doi: 10.1016/j.electacta.2004.02.036 [Crossref] [ Google Scholar]
- Arqiani M, Jonidi Jafari A, Rezaeei Kalantary R, Gholami M. Study of the aniline removal from industrial wastewater by electrochemical process. Iran Occupational Health 2013;10(1):70-8. [Persian].
- İrdemez Ş, Demircioğlu N, Yıldız YŞ, Bingül Z. The effects of current density and phosphate concentration on phosphate removal from wastewater by electrocoagulation using aluminum and iron plate electrodes. Sep Purif Technol 2006; 52(2):218-23. doi: 10.1016/j.seppur.2006.04.008 [Crossref] [ Google Scholar]
- Footemi M, Kholghi M, Hoorfar AH, Haghshenas D. Laboratory study on arsenic removal effect of iron nanoparticles in aqueous media. J Environ 2012; 39(4):149-56. [ Google Scholar]
- Brandhuber P, Amy G. Alternative methods for membrane filtration of arsenic from drinking water. Desalination 1998; 117(1-3):1-10. doi: 10.1016/s0011-9164(98)00061-7 [Crossref] [ Google Scholar]
- Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 2002; 17(5):517-68. doi: 10.1016/s0883-2927(02)00018-5 [Crossref] [ Google Scholar]
- Mollah MY, Pathak SR, Patil PK, Vayuvegula M, Agrawal TS, Gomes JA. Treatment of orange II azo-dye by electrocoagulation (EC) technique in a continuous flow cell using sacrificial iron electrodes. J Hazard Mater 2004; 109(1-3):165-71. doi: 10.1016/j.jhazmat.2004.03.011 [Crossref] [ Google Scholar]
- Sun X, Doner HE. An investigation of arsenate and arsenite bonding structures on goethite by FTIR. Soil Sci 1996; 161(12):865-72. [ Google Scholar]
- Manning BA, Fendorf SE, Goldberg S. Surface structures and stability of arsenic(III) on goethite: spectroscopic evidence for inner-sphere complexes. Environ Sci Technol 1998; 32(16):2383-8. doi: 10.1021/es9802201 [Crossref] [ Google Scholar]
- Idhayachander R, Palanivelu K. Electrolytic recovery of nickel from spent electroless nickel bath solution. E-J Chem 2010; 7(4):1412-20. doi: 10.1155/2010/216807 [Crossref] [ Google Scholar]