Avicenna J Environ Health Eng. 10(1):1-9.
doi: 10.34172/ajehe.2023.5295
Original Article
The Burden of Diseases From Exposure to Environmental Cigarette Smoke: A Case Study of Municipal Staff in Qazvin, Iran
Ali Safari Variani 1  , Zohreh Yazdi 2, Zahra Hosseinkhani 1, Javad Abbas Alimadadi 3, Masoumeh Ziaeiha 1, Hamid Karyab 1, *
, Zohreh Yazdi 2, Zahra Hosseinkhani 1, Javad Abbas Alimadadi 3, Masoumeh Ziaeiha 1, Hamid Karyab 1, * 
Author information:
1Research Center for Social Determinants of Health, Qazvin University of Medical Sciences, Qazvin, Iran
2Metabolic Diseases Research Center, Qazvin University of Medical Sciences, Qazvin, Iran
3Health Care Centre, Qazvin Municipality, Qazvin, Iran
Abstract
This study aimed to estimate the burden of diseases (BoD) from environmental cigarette smoke (ECS) exposure. More precisely, the study examined the prevalence of non-communicable diseases (NCDs) related to cigarette smoking in municipal staff based on a prospective cohort study. This cross-sectional study was designed among municipal employees, aged 25-55 years in Qazvin, Iran during 2019-2020. The data on cigarette smoking and environmental exposure were obtained using a standard questionnaire. Finally, systolic blood pressure (SBP) and diastolic blood pressure (DBP), fasting blood sugar (FBS), triglyceride (TG), high-density lipoprotein, and low-density lipoprotein were measured to assess the relation between active cigarette smoking and the prevalence of NCDs, including hypertension and diabetes in workers. The prevalence of cigarette smoking was 16.2%. In addition, 15% of staff were exposed to ECS. The prevalence of anemia and high TG levels in current cigarette smokers was 2.71 (P=0.024) and 1.4 times higher than among non-smokers (P=0.027). The total number of disability-adjusted life years (DALYs) caused by lung cancer, asthma, and ischemic heart disease (IHD) attributable to ECS was 0.65 per 1000 adults annually. Further, the number of 0.058 death was estimated per 1000 adults annually at the workplace. Most deaths were caused by IHD (79%), followed by lung cancer (12%) and asthma (9%). It was revealed that the number of DALYs and deaths attributable to secondhand smoke (SHS) was 0.34 and 0.3 vs. 0.028 and 0.029 per 1000 adults in men and women, respectively. The results demonstrated that exposure to ECS is an important factor in increasing the risk of the prevalence of NCDs and can increase the BoD attributable to cigarette smoking.
Keywords: Burden of diseases, DALY, Environmental cigarette smoke, Municipal staff,
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Safari Variani A, Yazdi Z, Hosseinkhani Z, Abbas Alimadadi J, Ziaeiha M, Karyab H. The burden of diseases from exposure to environmental cigarette smoke: a case study of municipal staff in Qazvin, Iran. Avicenna J Environ Health Eng. 2023; 10(1):1-9. doi:10.34172/ajehe.2023.5295
1. Introduction
Secondhand smoke (SHS) is designated as environmental tobacco smoke and classified among principal exposures to indoor air pollution. SHS comprises tobacco smoke, exhaled gases from smokers’ lungs, and the smoke generated due to the burning of cigarette contents (1,2). Nevertheless, smoke-free laws were implemented to reduce SHS exposure in public buildings, government offices, and workplaces (3,4). Various studies demonstrated that SHS is still a risk factor to threaten human health in workplaces. For instance, Tripathy reported that exposure to SHS in government offices was 21.2% in India (5). Likewise, Schneider et al demonstrated a prevalence of 9.9% (95% confidence interval [CI]: 8.3-11.5) of SHS exposure at workplaces in European countries (1). Moreover, the pooled prevalence of SHS exposure was reported 0.29 (95% CI: 0.23-0.36) by Rashiden et al in a systematic review and meta-analysis (6). Furthermore, investigations revealed 1.22 million deaths due to SHS exposure throughout the world in 2017 (7). In adults, SHS exposure has turned out to be related to multiple unfavorable health outcomes. In this study, it was assumed that the results from formerly studies are adequate to a cause-and-effect proof between SHS exposure and health-related problems (including lung cancer, ischemic heart disease [IHD], and asthma) (8). Caliri et al and Besaratinia & Pfeifer also reported the results of smoking in the generation of reactive oxygen and nitrogen with a potential oxidative damage increase to macromolecular targets which may lead to cancer initiation and lung carcinogenesis (9,10). The odds ratios (OR) for severe dyspnoea and asthma increased as a function of SHS exposure evaluated by Korsbæk et al (11). On the other hand, it should be noted that active smoking directly affects human health, and these adverse effects can be assessed as non-communicable diseases (NCDs). NCDs comprise an ample amount of medical conditions that are chronic in nature, and in general, progress gradually; consequently, such conditions are named chronic diseases (12). NCDs are the leading cause of morbidity and fatalities, especially in low- and middle-income countries (13-15). According to the published report by the World Health Organization (WHO), 36 out of 56 million global deaths were caused by NCDs with the majority of cardiovascular diseases, cancers, diabetes, and chronic lung diseases. Further, in this report, it was indicated that smoking is among the main risk factors which can cause NCDs (16). Cigarette smoking belongs to one risk factor accustomed to the four primary groups of NCDs which comprise IHD, cancer, chronic pulmonary disease, and diabetes. It was estimated that by 2020 and 2030, tobacco will kill over 7.5 and 8 million people worldwide (16,17). In addition, it provokes a minimum of 16 various kinds of cancers that are highly linked with lung cancer, which is the foremost cause of cancer deaths globally, calculating about one in five cancer deaths (18). Moreover, smoking is known to cause several cancers, including the upper esophagus, stomach, bladder, kidneys, colorectal, prostate, and pancreas (19).
Municipalities, as a workplace and non-governmental public organizations (20), have a central role in ensuring the provision of social, cultural, training, and health care to citizens. Therefore, paying attention to the levels of health and well-being of municipal staff is highly important (21). The results from searching in Scopus, Web of Science, PubMed, and Google Scholar databases represented that no study has so far reported the burden of diseases (BoD) (22) attributable to exposure to SHS among municipal staff. Accordingly, this study sought to provide insight into the health impact of SHS exposure, including lung cancer, asthma, and IHD, on staff in municipalities from Qazvin, Iran. In addition, this study was designed to evaluate the relationship between active smoking and hypertension, diabetes, blood lipid disorders, and anemia based on a prospective cohort study among municipal staff in Qazvin, Iran.
2. Methods
The present cross-sectional study was designed among total municipal employees, including 1225 people aged 25-55 years in Qazvin, Iran. The investigation was authorized by the Ethics Committee of the Qazvin University of Medical Sciences (No. IR.QUMS.REC.1397.222), and the protocol of the study was fulfilled with the Helsinki Declaration of 1975 (revised in 2008).
2.1. Data Gathering Using a Standard Questionnaire
A standard tool named the “WHO-stepwise Approach Surveillance Questionnaire” was used for collecting information from municipal staff (23). The obtained data comprised population-based parameters such as gender, age, tobacco, cigarette smoking, and SHS exposure and were collected by a trained team including four interviewers and supervisors. The consent was obtained by informing each staff as well. Non-smokers were defined as staff who had never smoked, while current smokers (active smoking) were defined as participants who had been smoking for more than 1 year. In addition, they were asked whether they had been exposed to tobacco smoke on duty during the previous year. If the response was positive, they were categorized as the group exposed to SHS. The double categorization of exposure was used to divide the employees into SHS exposed and unexposed groups. Those who were exposed to SHS had colleagues who smoke, and/or working in the room with customers who smoke during 8 hours working at the workplace (24). Anthropometric entities including height, weight, and waist circumference, were measured to brief participants’ physical status by utilizing standard approaches. Moreover, weight was determined using a solar-powered digital scale with attaining accuracy of ± 100 g. Additionally, the height was assessed in field circumstances by applying a changeable measuring board made up of wood. Finally, the gathered information was employed to assess the burden of lung cancers, asthma, and IHD referable to SHS exposure at the workplace.
2.2. Assessment of Non-communicable Diseases
This section was arranged to evaluate the relation between active cigarette smoking and the prevalence of NCDs, including hypertension and diabetes, in the municipal staff. Previous studies reported that cigarette smoking is associated with blood lipid disorders and anemia (25,26). Therefore, health adverse effects attributed to active smoking, including blood lipid disorders, anemia, hypertension, and diabetes, were investigated among municipal staff. After taking a rest for five to ten minutes in a sitting position, blood pressure was measured in the right arm by using an Omron Digital Blood Pressure machine (Omron M2 automatic BP monitor, Tokyo, Japan). Systemic hypertension for participants was defined as a measurement of systolic blood pressure (SBP) equal to/more than 140 mm Hg or diastolic blood pressure (DBP) equal to/more than 100 mm Hg (27). Furthermore, a formerly identified condition and/or the intake of anti-hypertensive drugs, irrespective of the BP interpretations, was regarded as hypertension. Following nightlong fasting of ≥ 12 hours before the interview, the blood samples (10 mL) were gathered in the morning to measure fasting blood sugar (FBS), hemoglobin (Hb), and blood lipids, including triglyceride (TG) (28), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol. By performing high-speed centrifugation, the serum was collected and immediately analyzed. The values of FBS were obtained by employing the enzymatic method using Eliteh Kit (Hitachi Machine, Feppim, French). To assess the anemia status, blood Hb levels were analyzed through the turbidimetric inhibition immunoassay method using a German Roche kit. Hb levels were categorized as Hb equal to/more than 13.0 g/dL in men (not anemic) and Hb equal to/more than 12 g/dL in women (29). The enzymatic method was used to measure TG using commercial kits. HDL was determined by the precipitation of phosphotungstic acid MgCl, and LDL was then calculated as well. Impairment fasting glucose was defined as ≥ 100 mg/dL and < 126 mg/dL (30).
2.3. Estimation of BoD
The burden of disease among non-smokers (DALYns) was calculated using Eq. (1), where DALYtotal is an absolute disease burden between smokers and non-smokers. In addition, PAFsm denotes the population derivative fraction associated with active smoking, which was computed using Eq. (3). Further, Ps represents the smoking prevalence, which was adopted from the questionnaire survey in a previous study (8).
(1)
Next, the burden derived from SHS exposure within non-smokers (population attributable fraction: PAFSHS) was analyzed using Eq. (2) as follows (8):
(2)
Furthermore, Eq. (3) was employed to calculate the PAF for individual health results.
where p and RR indicate the population proportion in a particular age group exposed to SHS and the relative risk for the outcomes of health effects, respectively (8).
(3)
By summing up all the attributable health effects in the specified workers, Eqs. 1 to 3 were used to calculate the total burden of deaths attributable to SHS exposure. The rate of exposure to SHS at work in municipal workers, by the percentage of men and women, was obtained from the questionnaire survey. Table 1 provides the relative risk and results related to the BoD among smokers and non-smokers at workplaces.
Table 1.
Relative Risk and Outcomes of Burden of Diseases Among Both Smokers and Non-smokers at Workplaces
|
Health Effects
|
Relative Risk (95% CI),
|
DALY/1000 Adults
|
Deaths/1000 Adults
|
|
Men
|
Women
|
Men
|
Women
|
| Adult asthma |
1.97 |
1.27 |
0.93 |
0.03 |
0.03 |
| Lung cancer |
1.21 |
1.85 |
0.77 |
0.21 |
0.08 |
| Ischaemic heart disease |
1.27 |
8.24 |
5.60 |
0.85 |
0.75 |
Note. CI: Confidence interval; DALY: Disability-adjusted life years.
In addition, the determination of both the past and present prevalence of SHS exposure was critical to estimate health effects due to chronic or acute diseases. Thus, for lung cancer that has several lags between exposure and effect (10 and 20 years), the average of long-term exposure data (past smoking) was employed to estimate the burden of deaths and disability-adjusted life years (DALYs). Of course, the interval between exposure and cardiovascular effects and asthma is extremely shorter; therefore, in these cases, it was assumed that current exposures were responsible for their BoD.
2.4. Data Analysis
Data were analyzed using SPSS statistical software, version 23 (SPSS Inc, Chicago Illinois, USA). The Kolmogorov-Smirnov test was applied to determine the normality of the distribution of variables. Moreover, the Student’s independent t-test and Mann-Whitney test for parameters were employed to compare smoking and gender groups with normal or non-normal distribution, respectively. A P value less than 0.05 was considered to determine statistically significant outcomes. Continuous and categorical variables were indicated by means ± standard deviations (SD) and percentages, respectively. Additionally, a logistic regression model was intended for the multivariate analyses of hypertension, blood lipid disorder, anemia, and diabetes due to cigarette smoking. Finally, the associations were analyzed by computing the OR with a 95% CI.
3. Results and Discussion
3.1. Descriptive Statistics
Table 2 presents the baseline characteristics of the participants. From 1254 invited people, the study population consisted of 956 individuals (11.9% women and 88.1% men). The mean age of participants was 38.54 years, and most of them were in the range of 31-40 years (51.6%). In addition, 77.5% and 90.7% of individuals had an academic education and were married, respectively. The proportion of performing walking in physical activity was dominant among participants so that the prevalence of walking was 4.76 and 4.40 times more than vigorous and moderate physical activity. The estimated body mass index (BMI) in 66.1% of men and 71.8% of women was more than 25 kg/m2. As a result, they were overweight or obese.
Table 2.
Baseline Characteristics of Municipal Staff in the Study
|
General Characteristics
|
Women and Men (n=956)
|
Men (n=842)
|
Women (n=114)
|
| Age (mean ± SD), year |
38.54 ± 7.6 |
38.67 ± 7.70 |
37.44 ± 6.59 |
| Physical activity |
|
|
|
| Vigorous physical activity (%) |
15.6 |
16.9 |
2.8 |
| Moderate physical activity (%) |
16.9 |
17.4 |
11.9 |
| Walking (%) |
74.4 |
76.3 |
6.1 |
| Height (Mean ± SD), cm |
173.8 ± 8.5 |
175.46 ± 7.56 |
161.19 ± 6.15 |
| Weight (Mean ± SD), kg |
80.83 ± 14.59 |
82.72 ± 14.03 |
67.29 ± 11.85 |
|
BMI (Mean ± SD), kg/m2
|
26.72 ± 4.39 |
26.86 ± 4.37 |
25.69 ± 4.46 |
3.2. Secondhand Smoking Exposure
Table 3 summarizes smoking behaviors and exposure parameters of municipal staff via SHS and active smoking. The prevalence of current tobacco smoking was 16.2%. Cigarette and hookah were the main routes of tobacco smoking in this study. Among the 956 participants, 12.9% (n = 123) were current smokers. Men were more likely to be current smokers than women (14.37% men vs. 1.75% women, P < 0.001). The age of starting cigarette smoking in 22% of participants was under 18 years, and 48% and 30% of them were in the age range of 18-25 and above 26 years old, respectively. Based on the results, 15% of staff were exposed to SHS. The results revealed that the prevalence of past and current cigarette smoking was 25.1 and 12.9%, which is comparable with many previous studies. In the study by Malta et al, the prevalence of past and current smokers in the adult population was reported 35.5% and 11.3%, respectively in Brazil (31). Similarly, Cornelius et al demonstrated that 12.5% of adults were current smokers in the United States (32). Despite control programs in the past decades, tobacco usage is widely reported in the world. According to the WHO, 22% of people aged over 15 years are smokers. Tobacco in India is frequently consumed by cigarette smoking, representing that approximately 30% of the Indian population aged > 15 years consumed a certain kind of tobacco in 2017 (33), and 19.3% of them used tobacco in the united states in 2018 (34). The low prevalence of current cigarette smoking among staff can be simultaneously due to the implementation of regular training programs in municipalities. Furthermore, the obtained results revealed that 77.5% of staff had academic education. Hence, it can reduce the prevalence of cigarette smoking (35,36). The results further represented that 14.8% and 16.48% of non-smoker men and women were exposed to SHS at the workplace, respectively, which is currently considered a risk factor for the advancement of health effects such as lung cancer, heart disease, and altered lung functions in passive smokers (37). The prevalence of SHS exposure among municipal staff in this study was lower than those of studies by Tsai et al (25.2%) in the United States (38) and Patten et al (20%) in the Canadian household population (21).
Table 3.
Smoking Behavior and Exposure Parameters of Respondents in This Study
|
Smoking Characteristics
|
Women and Men (n=956)
|
Men (n=842)
|
Women (n=114)
|
| Past smoking (%) |
25.1 |
24.76 |
0.34 |
| Cigarette (%) |
17.7 |
17.38 |
0.32 |
| Hookah (%) |
6.6 |
6.4 |
0.2 |
| Others (%) |
0.8 |
0.8 |
0.0 |
| Current smoking (%) |
16.2 |
15.8 |
0.4 |
| Cigarette (%) |
12.9 |
12.7 |
0.2 |
| Hookah (%) |
3.1 |
2.9 |
0.2 |
| Others (%) |
0.2 |
0.2 |
0.0 |
| Cigarette smoking pattern in current smokers (%) |
| 1-10 cigarettes/day |
83.6 |
82.6 |
100 |
| 11-20 cigarettes/day |
6.1 |
6.4 |
-- |
| > 20 cigarettes/day |
10.4 |
10.9 |
-- |
| Mean number of cigarettes per day |
22.8 |
23 |
5 |
| Proportion of non-smoking staff exposed to SHS (%) |
15 |
14.8 |
16.47 |
Note. SHS: Secondhand smoke.
3.3. The Prevalence of Chronic Diseases
Based on the data in Table 4, the age- and gender-adjusted prevalence of hypertension was 10.35% so that 99 participants had hypertension with SBP/DBP ≥ 140/100. The prevalence of high SBP and high DBP was 9.83% (146.49 ± 9.29 mm Hg) and 2.19% (102.72 ± 8.07 mm Hg), respectively. Likewise, the prevalence of diabetes was 4.39% (3.72% and 8.98% in non-smokers and cigarette smokers, respectively) with a mean of FBS 195.88 ± 7.93 mg/dL. The present results showed that 16.53% (158 persons) suffer from impaired glucose tolerance (7).
Table 4.
Prevalence of Chronic Diseases in the Municipal Staffs (%, number)
|
Parameters
|
Mean Levels in Patients
|
Unit
|
Criteria
|
Women and Men (n=956)
|
Men (n=842)
|
Women (n=114)
|
| Non-communicable diseases |
| Hypertension |
SBP: 146.49 ± 9.29
DBP: 102.72 ± 8.07
|
mm Hg |
SBP/DBP ≥ 140/100 |
10.35 (99) |
11.40 (96) |
2.63 (3) |
| Diabetes |
195.88 ± 7.93 |
mg/dL |
FBS > 126 |
4.39 (42) |
4.99 (42) |
Not detected |
| Blood lipid disorders |
| High TG levels |
208 ± 3.29 |
mg/dL |
TG > 199 |
20.82 (199) |
22.21 (187) |
10.53 (12) |
| Low HDL levels |
Men: 30.27 ± 7.16
Women: 40.54 ± 6.77
|
mg/dL |
Men: HDL < 40 Women: HDL < 50 |
60.67 (580) |
53.13 (508) |
63.14 (72) |
| High LDL levels |
177.67 ± 20.59 |
mg/dL |
LDL > 160 |
1.34 (13) |
1.54 (13) |
Not detected |
| Anemia |
Men: 11.77 ± 1.87
Women: 11.44 ± 0.28
|
g/dL |
Men: Hb< 13.0 Women: Hb< 12.0 |
5.33 (51) |
2.14 (18) |
27.19 (31) |
Note. SBP: Systolic blood pressure; DBP: Diastolic blood pressure; TG: Triglyceride; HDL: High-density lipoprotein level; LDL: Low-density lipoprotein level; FBS: Fasting blood sugar; Hb: Hemoglobin.
Table 5 compares the risk factors between smokers and non-smokers, indicating a statistically significant increase in FBS in smokers. The remaining parameters were not statistically significant. The analysis of gender differences shows statistically significant differences in pulse pressure, heart rate, SBP, DBP, TG levels, LDLs, Hb levels, and BMI. Globally, the foremost provoked condition of NCD is cardiovascular disease, whereas the leading remediable risk factor for cardiovascular disease is hypertension, which annually causes more than 9 million deaths worldwide (39).
Table 5.
Comparison of Risk Factors Between Cigarette Smoking and Gender (Mean ± SD)
|
Risk Factors
|
Non-smokers (n=833)
|
Smokers (n=123)
|
P
value
|
Men (n=842)
|
Women (n=114)
|
P
value
|
| Pulse pressure (mm Hg) |
45.84 ± 7.18 |
46.18 ± 9.76 |
0.768 |
45.32 ± 9.22 |
42.09 ± 7.46 |
0.000 |
| Heart rate (beats per minute) |
80.74 ± 5.36 |
82.18 ± 4.82 |
0.277 |
82.03 ± 4.94 |
82.05 ± 6.16 |
0.000 |
| SBP (mm Hg) |
121.66 ± 10.94 |
122.89 ± 14.08 |
0.959 |
121.96 ± 12.13 |
114.77 ± 10.53 |
0.000 |
| dBP (mm Hg) |
75.82 ± 8.81 |
76.72 ± 8.71 |
0.793 |
76.54 ± 8.34 |
72.68 ± 7.39 |
0.000 |
| TG levels (mg/dL) |
165.41 ± 111.27 |
170.66 ± 114.02 |
0.661 |
174.67 ± 155.56 |
118.44 ± 7.31 |
0.000 |
| HDL(mg/dL) |
37.98 ± 11.00 |
34.37 ± 10.05 |
0.733 |
36.47 ± 12.21 |
53.95 ± 97.83 |
0.294 |
| LDL(mg/dL) |
104.87 ± 23.88 |
102.97 ± 26.53 |
0.017 |
103.97 ± 23.89 |
102.03 ± 21.42 |
0.000 |
| HB levels (g/dl) |
15.91 ± 1.34 |
15.88 ± 1.33 |
0.679 |
15.87 ± 1.31 |
13.59 ± 1.44 |
0.000 |
| FBS (mg/dL) |
92.05 ± 28.64 |
101.63 ± 48.28 |
0.000 |
94.39 ± 31.52 |
87.26 ± 9.65 |
0.014 |
|
BMI (kg/m2)
|
26.77 ± 4.01 |
26.96 ± 6.28 |
0.488 |
26.78 ± 4.36 |
26.04 ± 4.39 |
0.008 |
Note. SD: Standard deviation; SBP: Systolic blood pressure; TG: Triglyceride; DBP: Diastolic blood pressure; TG: Triglyceride; BMI: Body mass index; LDL: low-density lipoprotein; HDL: high-density lipoprotein; FBS: Fasting blood sugar; Hb: Hemoglobin.
The hypertension prevalence in this study (10.35%) was lower than that the one reported among office workers in a multi-national company in the Niger Delta (25.9%) in 2019 (40), workers at Hawassa University, Ethiopia (19.7%) in 2018 (41), and among iron and steel foundry current smoker workers (75.8%) in 2019 (42). The logistic regression analysis (Table 6) revealed that the prevalence of hypertension in smokers and non-smokers was not statistically significant (P = 0.23). Similarly, Primatesta et al reported that cigarette smoking does not have a significant influence on blood pressure (43). Moreover, the findings of a prospective cohort study demonstrated that current smoking was positively associated with hypertension in Kingston (44). Likewise, a strong association (OR = 1.49, IC = 0.76-2.93) was found between smoking and arterial hypertension in a literature review (45). Consequently, pulse pressure monitoring represented that even though more than 10% of participants had hypertension, cigarette smoking alone was not a significant factor in high blood pressure.
Table 6.
Logistic Regression Analysis of Noninvasive Diseases in Two Groups of Smokers and Non-smokers After Adjusting the Effect of Age and Gender
|
NCDs
|
Yes
|
Number (%) in Smokers
|
Number (%) in Non-smokers
|
OR (95% CI)
|
P
value
|
| Hypertension |
No |
21 (17.07) |
78 (9.36) |
0.85 (0.481-1.141) |
0.23 |
| Yes |
102 (82.93) |
755 (90.64) |
|
|
| Anemia |
No |
2 (1.63) |
49 (5.88) |
2.71 (1.139-6.47) |
0.024 |
| High triglyceride |
Yes |
121 (98.37) |
784 (94.12) |
|
|
| No |
33 (26.83) |
66 (7.92) |
1.4 (1.04-1.88) |
0.027 |
| Fast blood sugar |
Safe |
90 (73.17) |
767 (92.08) |
|
|
| ITG |
88 (71.54) |
668 (80.19) |
0.566 (0.38-0.84) |
0.005 |
| Diabetic |
24 (19.51) |
134 (16.09) |
|
|
|
|
11 (8.94) |
31 (3.72) |
|
|
Note. NCD: Non-communicable disease; ITG: Impaired glucose tolerance; OR: Odds ratio; CI: Confidence interval.
Iron deficiency anemia is another public health problem around the world, especially in developing countries. It affects maternal and child mortality, physical performance, and referral to healthcare professionals and occurs in absolute or functional forms (46). Furthermore, it is known as a diminution in ≥ 1 major red blood count measurement which can be associated with numerous chronic diseases that can be complicated by anemia (47). Based on the results of logistic regression analysis (Table 6), the rate of anemia in non-smokers was 2.71 times higher than in smokers (P = 0.024). Cigarette smoking caused an increase in the Hb level. The attachment of carbon monoxide with Hb results in the formation of carboxy-Hb, leading to a diminution in the capacity of Hb to supply oxygen to the tissues. The compensatory mechanism will get activated because of decreased oxygen delivery, and smokers will maintain a higher Hb level than non-smokers (47,48). Similarly, Yun et al and Nasimi et al emphasized that the mean of Hb was significantly higher in smokers than in non-smokers (49,50). Hence, low Hb levels cannot be attributed to cigarette smoking, and other risk factors affecting anemia (e.g., diet) should be assessed among municipal staff (51,52).
As another public health problem, diabetes is a group of NCDs, resulting from high blood sugar levels over a prolonged period. Generally, it can be categorized into type 1, type 2, and gestational diabetes mellitus while specifically into neonatal and maturity-onset diabetes (30). In this study, 8.94% of current smokers had diabetes with FBS > 126 mg/dL, which was comparable with 6.6% in Iranian adults aged 20-70 years (6.6%) in another study (53). In addition, the results of logistic regression analysis revealed that the chance of healthy FBS in smokers was 0.56 than in non-smokers (P = 0.005). Among the general population, prospective investigations represented that cigarette smoking is linked with a high risk for diabetes mellitus in both males and females (54). Another study reported that 12% of the diabetes incidence in the USA was associated with smoking (22). A low prevalence of diabetes in municipal staff (8.94% in smokers vs. 3.72% in non-smokers) can be based on specific reasons. In this study, it was found that many diabetics were in control and were taking medication. Consequently, the reported prevalence of diabetes can be underestimated. Further, given that several staff (16.5% of municipal staff; 19.5% in smokers vs. 16.1% in non-smokers) were at the stage of impaired glucose tolerance (7), care implementation plans to prevent diabetes are necessary. Elevated triglycerides and blood fats are the other risk factors that can greatly increase the risk of cardiovascular diseases at concentrations of 2-10 mmol/L and > 10 mmol/L, deliberating a high chance of acute pancreatitis and might be of cardiovascular disease (28). The other forms of blood fats, including HDL and LDL levels, are proven to be under the influence of cigarettes. HDL is strongly and inversely related to cigarette smoking in both males and females (28,55). The results of logistic regression analysis showed that the chances of high triglycerides in smokers were 1.4 times higher than in non-smokers (P = 0.027), which is in concordance with the findings of Waqar, indicating that cigarette smoking provokes modification in blood lipid concentrations in the direction of raised risk for coronary artery disease (56). In this survey, it was revealed that a high TG level in 10.3% of participants can act as a risk factor for heart disease. Moreover, it was demonstrated that cigarette smoking can lead to an increased risk of heart disease due to high TG levels (26.8% in smokers vs. 7.92% in non-smokers).
3.4. BoD
Table 7 provides the outcomes of the BoD, including DALYs and deaths attributable to SHS exposure in municipal staff at the workplace. The average PAF due to SHS was 5.3%, which ranged from 2.5% to 10.7%. The number of DALYs caused by lung cancer, asthma, and IHD attributable to SHS was estimated at 0.02-0.21, and a total of 0.65 per 1000 adults annually at the workplace. Further, the number of deaths was estimated at 0.002-0.024, and a total of 0.058 death per 1000 adults annually at the workplace.
Table 7.
DALYs and Deaths Attributable to SHS Exposure at Workplace in This Study
|
Health Effects
|
PAF for SHS
|
DALY/1000 Employee
|
Deaths/1000 Employees
|
|
Men
|
Women
|
Men
|
Women
|
Men
|
Women
|
| Lung cancer |
0.025 |
0.027 |
0.036 |
0.020 |
0.004 |
0.002 |
| IHD |
0.030 |
0.032 |
0.209 |
0.181 |
0.022 |
0.024 |
| Asthma |
0.100 |
0.107 |
0.100 |
0.100 |
0.003 |
0.003 |
Note. DALY: Disability-adjusted life years; SHS: Secondhand smoke; PAF: Population attributable fraction; IHD: Ischemic heart disease.
The total number of DALYs caused by lung cancer, asthma, and IHD attributable to SHS was estimated at approximately 0.647 annually per 1000 employees at the workplace. The highest disease burden was estimated for IHD (0.345 DALYs per 1000 workers and 60% DALYs), asthma (31% DALYs), and lung cancer (9% DALYs), respectively. The DALYs calculate the years of life lost and those lived with disability caused by SHS-related diseases. As illustrated in Fig. 1, the DALYs of lung cancer, IHD, and asthma attributable to SHS represent approximately 2.5, 3, and 10 % of DALYs attributable to non-smokers in men, respectively. These ratios were 2.6, 3.2, and 10.7% in women.
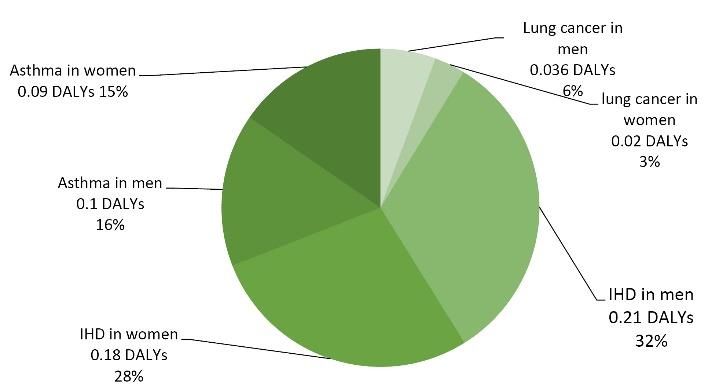
Fig. 1.
Distribution of Annually DALYs per 1000 Employees at Workplace Attributable to Secondhand Smoke Exposure. Note. DALY: Disability-adjusted life years; IHD: Ischemic heart disease
.
Distribution of Annually DALYs per 1000 Employees at Workplace Attributable to Secondhand Smoke Exposure. Note. DALY: Disability-adjusted life years; IHD: Ischemic heart disease
Fig. 2 displays the distribution of total deaths attributable to SHS exposure. It was found that the resulting disease burden of asthma, lung cancer, and IHD attributable to SHS amounts to 0.052 deaths annually per 1000 employees at the workplace. Therefore, considering 30 years of work before retirement, 1.5 deaths are attributable to SHS exposure in municipal staff. Most of these deaths are caused by IHD (79%), followed by lung cancer (12%) and asthma (9%).
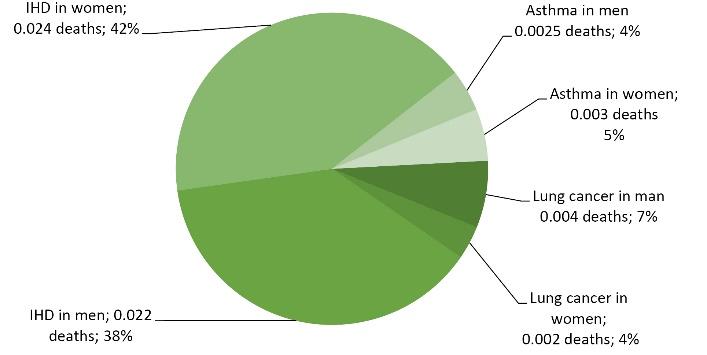
Fig. 2.
Distribution of Annually Total Deaths per 1000 Employees Attributable to Secondhand Smoke Exposure. Note. IHD: Ischemic heart disease
.
Distribution of Annually Total Deaths per 1000 Employees Attributable to Secondhand Smoke Exposure. Note. IHD: Ischemic heart disease
Based on the data in Figs. 1 and 2, there are pronounced health effect differences in the burden of the different burden, especially in health effects attributed to IHD, which seems to have the highest impact on the burden of DALYs and deaths attributable to SHS exposure in workers in the municipality.
Stacked columns (Fig. 3) were used to present the composition of DALYs and deaths in men and women, showing pronounced differences in the assessed variables attributed to SHS exposure. As shown, deaths in men, deaths in women, and DALYs in men had the highest share in the composition of lung cancer, IHD, and asthma, respectively. Our finding revealed that the number of DALYs attributable to SHS was 0.34 (53.5%) and 0.3 (46.5%) per 1000 employees in men and women, respectively. Moreover, the number of deaths attributable to SHS was 0.028 (48.94%) and 0.029 (51.06%) per 1000 employees in men and women, respectively. Therefore, there were no pronounced differences in gender ratios, which contradicts the findings of Carreras et al, representing that the BoD from secondhand tobacco smoke exposure among adults from European Union countries in men was higher than in women (57). A similar finding was reported by Sugiharto et al, evaluating the value of DALY stroke in Indonesia (58). This study had several limitations. First, we only found a detailed relation between cigarette smoking and NCDs. Therefore, it cannot account for the effects of other risk factors on the prevalence of NCDs, including alcohol, nutrition, and physical activity, which is supported by previous studies (59,60). In addition, this study was performed based on a cross-sectional design with a single study visit. Further, although the relative risks employed in PAF calculations were the foremost appraisal of the association between exposure to SHS and the incidence of diseases, it may be subject to random and systematic errors. On the other hand, the study had several strengths. First, the determination of blood tests such as Hb, lipids, and fasting plasma glucose levels for all staff provided a large sample size for analysis. Furthermore, questioning and completing the collection forms were conducted by experts. Finally, the gathered data can be used for various types of research, especially in cohort studies.
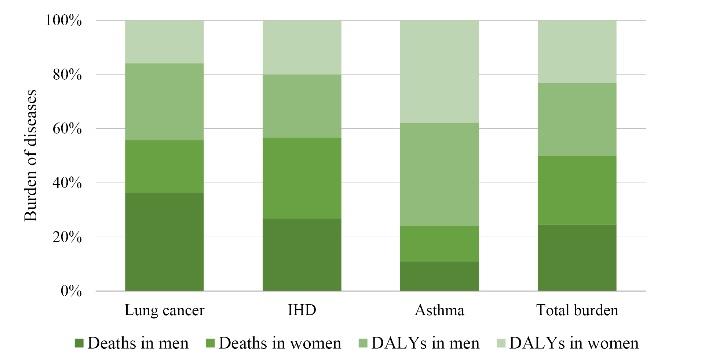
Fig. 3.
Ratios of Burden of Deaths and DALYs Attributable to SHS Exposure in Different Genders. Note. DALY: Disability-adjusted life years; IHD: Ischemic heart disease; SHS: Secondhand smoke
.
Ratios of Burden of Deaths and DALYs Attributable to SHS Exposure in Different Genders. Note. DALY: Disability-adjusted life years; IHD: Ischemic heart disease; SHS: Secondhand smoke
4. Conclusion
Overall, the prevalence of cigarette smoking was 16.2%, thus 15% of staff were exposed to environmental cigarette smoke (ECS). The total number of DALYs caused by lung cancer, asthma, and IHD attributable to ECS was 0.65 per 1000 adults annually, and the contribution of men and women was 0.34 and 0.31, respectively. The obtained results demonstrated that there were pronounced differences between the values of health impacts (lung cancer, asthma, and IHD) that were attributed to SHS exposure, but there were no significant differences in terms of gender. Moreover, the number of 0.058 death was estimated per 1000 adults annually and 1.5 deaths during 30 years at the workplace. Most of the deaths were due to IHD (79%), lung cancer (12%), and asthma (9%), respectively. Based on the findings, exposure to ECS is an important factor in increasing the risk of the prevalence of NCDs and can increase the BoD attributable to cigarette smoking.
Acknowledgments
The authors are grateful to the Vice-president for Research at Qazvin University of Medical Sciences and Officials of Qazvin Municipality for financial support.
Authors’ Contribution
Conceptualization: Hamid Karyab, Ali Safari Variani.
Data curation: Hamid Karyab, Zahra Hosseinkhani.
Formal analysis: Hamid Karyab, Ali Safari Variani, Zohreh Yazdi.
Funding acquisition: Hamid Karyab.
Investigation: Hamid Karyab, Masoumeh Ziaeiha, Javad Abbas Alimadadi, Ali Safari Variani, Zohreh Yazdi.
Methodology: Hamid Karyab, Ali Safari Variani, Zohreh Yazdi.
Project administration: Hamid Karyab, Javad Abbas Alimadadi.
Resources: Hamid Karyab, Zahra Hosseinkhani.
Supervision: Hamid Karyab, Ali Safari Variani, Zohreh Yazdi.
Validation: Zahra Hosseinkhani,Zohreh Yazdi.
Writing–original draft: Hamid Karyab, Zahra Hosseinkhani.
Writing–review & editing: Hamid Karyab, Zahra Hosseinkhani.
Competing Interests
The authors declare that they have no conflict of interests.
Funding
This study was supported by Qazvin Municipality and the Vice-president for Research at Qazvin University of Medical Sciences.
References
- Schneider S, Lunau T, Eikemo TA, Kotz D, Bambra C, Kuntz B. Better air but not for all? Changes in second-hand smoke exposure at workplaces in 29 European countries over 10 years. Eur J Public Health 2021; 31(4):708-14. doi: 10.1093/eurpub/ckab035 [Crossref] [ Google Scholar]
- Rafieyan S, Sadighi Shamami M, Shateri V, Ghojazadeh M, Salehnia F. Correlation of exposure to environmental tobacco smoke and periodontitis in passive smokers; a systematic review. Immunopathol Persa 2022; 8(2):e29308. doi: 10.34172/ipp.2022.29308 [Crossref] [ Google Scholar]
- Sansone G, Fong GT, Meng G, Craig LV, Xu SS, Quah ACK. Secondhand smoke exposure in public places and support for smoke-free laws in Japan: findings from the 2018 ITC Japan Survey. Int J Environ Res Public Health 2020; 17(3):979. doi: 10.3390/ijerph17030979 [Crossref] [ Google Scholar]
- Jankowski M, Rees V, Zgliczyński WS, Kaleta D, Gujski M, Pinkas J. Self-reported secondhand smoke exposure following the adoption of a national smoke-free policy in Poland: analysis of serial, cross-sectional, representative surveys, 2009-2019. BMJ Open 2020; 10(9):e039918. doi: 10.1136/bmjopen-2020-039918 [Crossref] [ Google Scholar]
- Tripathy JP. Secondhand smoke exposure at home and public places among smokers and non-smokers in India: findings from the Global Adult Tobacco Survey 2016-17. Environ Sci Pollut Res Int 2020; 27(6):6033-41. doi: 10.1007/s11356-019-07341-x [Crossref] [ Google Scholar]
- Rashiden I, Ahmad Tajuddin N, Yee A, Zhen STE, Bin Amir Nordin AS. The efficacy of smoking ban policy at the workplace on secondhand smoking: systematic review and meta-analysis. Environ Sci Pollut Res Int 2020; 27(24):29856-66. doi: 10.1007/s11356-020-09407-7 [Crossref] [ Google Scholar]
- Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392(10159):1923-94. doi: 10.1016/s0140-6736(18)32225-6 [Crossref] [ Google Scholar]
- Öberg M, Prüss-Üstün A, Schweizer C, Woodward A. Second hand smoke, assessing the environmental burden of disease at national and local levels. World Health Organization, Environmental burden of disease series, No 18. 2010. Available from: https://estia.hua.gr/file/lib/default/data/19785/theFile.
- Caliri AW, Tommasi S, Besaratinia A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat Res Rev Mutat Res 2021; 787:108365. doi: 10.1016/j.mrrev.2021.108365 [Crossref] [ Google Scholar]
- Besaratinia A, Pfeifer GP. Second-hand smoke and human lung cancer. Lancet Oncol 2008; 9(7):657-66. doi: 10.1016/s1470-2045(08)70172-4 [Crossref] [ Google Scholar]
- Korsbæk N, Landt EM, Dahl M. Second-hand smoke exposure associated with risk of respiratory symptoms, asthma, and COPD in 20,421 adults from the general population. J Asthma Allergy 2021; 14:1277-84. doi: 10.2147/jaa.s328748 [Crossref] [ Google Scholar]
- Bennett JE, Stevens GA, Mathers CD, Bonita R, Rehm J, Kruk ME. NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 34. Lancet 2018; 392(10152):1072-88. doi: 10.1016/s0140-6736(18)31992-5 [Crossref] [ Google Scholar]
- Kavishe B, Biraro S, Baisley K, Vanobberghen F, Kapiga S, Munderi P. High prevalence of hypertension and of risk factors for non-communicable diseases (NCDs): a population based cross-sectional survey of NCDS and HIV infection in Northwestern Tanzania and Southern Uganda. BMC Med 2015; 13:126. doi: 10.1186/s12916-015-0357-9 [Crossref] [ Google Scholar]
- Abdul Rahim HF, Sibai A, Khader Y, Hwalla N, Fadhil I, Alsiyabi H. Non-communicable diseases in the Arab world. Lancet 2014; 383(9914):356-67. doi: 10.1016/s0140-6736(13)62383-1 [Crossref] [ Google Scholar]
- Shariful Islam SM, Dannemann Purnat T, Phuong NT, Mwingira U, Schacht K, Fröschl G. Non-communicable diseases (NCDs) in developing countries: a symposium report. Global Health 2014; 10:81. doi: 10.1186/s12992-014-0081-9 [Crossref] [ Google Scholar]
- World Health Organization (WHO). Global Status Report on Noncommunicable Diseases 2014: Attaining the Nine Global Noncommunicable Diseases Targets; A Shared Responsibility. WHO; 2014. Available at: https://apps.who.int/iris/bitstream/handle/10665/148114/9789241564854_eng.pdf.
- World Health Organization (WHO). Global Status Report on Noncommunicable Diseases 2010. WHO Library Cataloguing-in-Publication Data. WHO; 2011. Available at: https://apps.who.int/iris/bitstream/handle/10665/44579/9789240686458_eng.pdf.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127(12):2893-917. doi: 10.1002/ijc.25516 [Crossref] [ Google Scholar]
- Khani Y, Pourgholam-Amiji N, Afshar M, Otroshi O, Sharifi-Esfahani M, Sadeghi-Gandomani H. Tobacco smoking and cancer types: a review. Biomed Res Ther 2018; 5(4):2142-59. doi: 10.15419/bmrat.v5i4.428 [Crossref] [ Google Scholar]
- Rostami V, Ameri M. Municipality in legal system of Iran and France. Comp Law Rev 2016; 7(1):141-61. doi: 10.22059/jcl.2016.58605 [Crossref] [ Google Scholar]
- Patten SB, Williams JVA, Lavorato DH, Woolf B, Wang JL, Bulloch AGM. Major depression and secondhand smoke exposure. J Affect Disord 2018; 225:260-4. doi: 10.1016/j.jad.2017.08.006 [Crossref] [ Google Scholar]
- Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007; 298(22):2654-64. doi: 10.1001/jama.298.22.2654 [Crossref] [ Google Scholar]
- World Health Organization (WHO). WHO STEPS Surveillance Manual: The WHO Stepwise Approach to Chronic Disease Risk Factor Surveillance. No. WHO/NMH/CHP/SIP/05.02. WHO; 2005. Available at: https://apps.who.int/iris/handle/10665/43376.
- Koda M, Kitamura I, Okura T, Otsuka R, Ando F, Shimokata H. The associations between smoking habits and serum triglyceride or hemoglobin A1c levels differ according to visceral fat accumulation. J Epidemiol 2016; 26(4):208-15. doi: 10.2188/jea.JE20150086 [Crossref] [ Google Scholar]
- Bhadarge G, Ambad R, Bankar N, Kotecha R. Study of cigarette smoking on haematological parameters and lipid profile in Vidharbha region, India. J Pharm Res Int 2021; 33(36A):213-17. doi: 10.9734/JPRI/2021/v33i36A31943 [Crossref] [ Google Scholar]
- Anandha Lakshmi S, Lakshmanan A, Ganesh Kumar P, Saravanan A. Effect of intensity of cigarette smoking on haematological and lipid parameters. J Clin Diagn Res 2014; 8(7):BC11-3. doi: 10.7860/jcdr/2014/9545.4612 [Crossref] [ Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003; 289(19):2560-72. doi: 10.1001/jama.289.19.2560 [Crossref] [ Google Scholar]
- Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet 2014; 384(9943):626-35. doi: 10.1016/s0140-6736(14)61177-6 [Crossref] [ Google Scholar]
- Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration?. Blood 2006; 107(5):1747-50. doi: 10.1182/blood-2005-07-3046 [Crossref] [ Google Scholar]
- American Diabetes Association. 2 Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care 2019; 42(Suppl 1):S13-S28. doi: 10.2337/dc19-S002 [Crossref] [ Google Scholar]
- Malta DC, Flor LS, Machado ÍE, Felisbino-Mendes MS, Brant LC, Ribeiro AL. Trends in prevalence and mortality burden attributable to smoking, Brazil and federated units, 1990 and 2017. Popul Health Metr 2020; 18(Suppl 1):24. doi: 10.1186/s12963-020-00215-2 [Crossref] [ Google Scholar]
- Cornelius ME, Loretan CG, Wang TW, Jamal A, Homa DM. Tobacco product use among adults - United States, 2020. MMWR Morb Mortal Wkly Rep 2022; 71(11):397-405. doi: 10.15585/mmwr.mm7111a1 [Crossref] [ Google Scholar]
- Arora V, Gupta N, Gupta P, Bansal M, Thakar S, Nagpal I. Cigarette smoking behavior and associated psychosocial determinants among school going adolescents in Panchkula, India. J Indian Assoc Public Health Dent 2017; 15(1):27-31. doi: 10.4103/2319-5932.201944 [Crossref] [ Google Scholar]
- Law SM, Lu X, Yu F, Tseng V, Law SK, Coleman AL. Cigarette smoking and glaucoma in the United States population. Eye (Lond) 2018; 32(4):716-25. doi: 10.1038/eye.2017.292 [Crossref] [ Google Scholar]
- Oikonomou MT, Arvanitis M, Sokolove RL. Mindfulness training for smoking cessation: a meta-analysis of randomized-controlled trials. J Health Psychol 2017; 22(14):1841-50. doi: 10.1177/1359105316637667 [Crossref] [ Google Scholar]
- La Torre G, D’Egidio V, Patrissi R, Chiarini M, De Vivo G, Mannocci A. Effectiveness of a training course on smoking cessation knowledge and behaviour for health profession students: the SISMA project. J Prev Med Hyg 2019; 60(2):E119-E23. doi: 10.15167/2421-4248/jpmh2019.60.2.1178 [Crossref] [ Google Scholar]
- Saha SP, Bhalla DK, Whayne TF Jr, Gairola C. Cigarette smoke and adverse health effects: an overview of research trends and future needs. Int J Angiol 2007; 16(3):77-83. doi: 10.1055/s-0031-1278254 [Crossref] [ Google Scholar]
- Tsai J, Homa DM, Gentzke AS, Mahoney M, Sharapova SR, Sosnoff CS. Exposure to secondhand smoke among nonsmokers - United States, 1988-2014. MMWR Morb Mortal Wkly Rep 2018; 67(48):1342-6. doi: 10.15585/mmwr.mm6748a3 [Crossref] [ Google Scholar]
- Angell SY, De Cock KM, Frieden TR. A public health approach to global management of hypertension. Lancet 2015; 385(9970):825-7. doi: 10.1016/s0140-6736(14)62256-x [Crossref] [ Google Scholar]
- Ofori SN, Obosi J. Prevalence of hypertension among office workers in a multi-national company in the Niger-Delta with the 2017 American College of Cardiology/American Heart Association Blood Pressure Guidelines. Prev Med Rep 2019; 15:100899. doi: 10.1016/j.pmedr.2019.100899 [Crossref] [ Google Scholar]
- Esaiyas A, Teshome T, Kassa D. Prevalence of hypertension and associate risk factors among workers at Hawassa University, Ethiopia: an institution based cross sectional study. J Vasc Med Surg 2018; 6(1):354. doi: 10.4172/2329-6925.1000354 [Crossref] [ Google Scholar]
- Shi P, Jing H, Xi S. Urinary metal/metalloid levels in relation to hypertension among occupationally exposed workers. Chemosphere 2019; 234:640-7. doi: 10.1016/j.chemosphere.2019.06.099 [Crossref] [ Google Scholar]
- Primatesta P, Falaschetti E, Gupta S, Marmot MG, Poulter NR. Association between smoking and blood pressure: evidence from the health survey for England. Hypertension 2001; 37(2):187-93. doi: 10.1161/01.hyp.37.2.187 [Crossref] [ Google Scholar]
- Doulougou B, Gomez F, Alvarado B, Guerra RO, Ylli A, Guralnik J. Factors associated with hypertension prevalence, awareness, treatment and control among participants in the International Mobility in Aging Study (IMIAS). J Hum Hypertens 2016; 30(2):112-9. doi: 10.1038/jhh.2015.30 [Crossref] [ Google Scholar]
- de Siqueira Galil AG, Cupertino AP, Banhato EF, Campos TS, Colugnati FA, Richter KP. Factors associated with tobacco use among patients with multiple chronic conditions. Int J Cardiol 2016; 221:1004-7. doi: 10.1016/j.ijcard.2016.07.041 [Crossref] [ Google Scholar]
- Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet 2016; 387(10021):907-16. doi: 10.1016/s0140-6736(15)60865-0 [Crossref] [ Google Scholar]
- Leifert JA. Anaemia and cigarette smoking. Int J Lab Hematol 2008; 30(3):177-84. doi: 10.1111/j.1751-553X.2008.01067.x [Crossref] [ Google Scholar]
- Malenica M, Prnjavorac B, Bego T, Dujic T, Semiz S, Skrbo S. Effect of cigarette smoking on haematological parameters in healthy population. Med Arch 2017; 71(2):132-6. doi: 10.5455/medarh.2017.71.132-136 [Crossref] [ Google Scholar]
- Yun SH, Choi YH, Moon YS, Ahn SH, Kim TG. Difference in hemoglobin between smokers and non-smokers. J Korean Acad Fam Med 2002; 23(1):80-6. [ Google Scholar]
- Nasimi A, Rostami A, Nematbakhsh M. Difference of blood’s oxygen between smokers and non-smokers patients with acute myocardial infarction and finding its cause in animal model. J Shahrekord Univ Med Sci 2005;7(1):14-20. [Persian].
- Zeng L, Pei L, Li C, Yan H. Iron deficiency anaemia. In: Khan J, ed. Current Topics in Anemia. IntechOpen; 2017. 10.5772/intechopen.69048.
- Petry N, Olofin I, Hurrell RF, Boy E, Wirth JP, Moursi M. The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: a systematic analysis of national surveys. Nutrients 2016; 8(11):693. doi: 10.3390/nu8110693 [Crossref] [ Google Scholar]
- Khalili-Moghadam S, Mirmiran P, Bahadoran Z, Azizi F. The Mediterranean diet and risk of type 2 diabetes in Iranian population. Eur J Clin Nutr 2019; 73(1):72-8. doi: 10.1038/s41430-018-0336-2 [Crossref] [ Google Scholar]
- Clair C, Cohen MJ, Eichler F, Selby KJ, Rigotti NA. The effect of cigarette smoking on diabetic peripheral neuropathy: a systematic review and meta-analysis. J Gen Intern Med 2015; 30(8):1193-203. doi: 10.1007/s11606-015-3354-y [Crossref] [ Google Scholar]
- Kosmas CE, Sourlas A, Guzman E, Kostara CE. Environmental factors modifying HDL functionality. Curr Med Chem 2022; 29(10):1687-701. doi: 10.2174/0929867328666210714155422 [Crossref] [ Google Scholar]
- Waqar A. Effects of tobacco smoking on the lipid profile of teenage male population in Lahore city. Int J Med Med Sci 2010; 2(6):172-7. doi: 10.5897/ijmms.9000082 [Crossref] [ Google Scholar]
- Carreras G, Lachi A, Cortini B, Gallus S, López MJ, López-Nicolás Á. Burden of disease from second-hand tobacco smoke exposure at home among adults from European Union countries in 2017: an analysis using a review of recent meta-analyses. Prev Med 2021; 145:106412. doi: 10.1016/j.ypmed.2020.106412 [Crossref] [ Google Scholar]
- Sugiharto M, Prayitno L. Effects of smokers and second hand smoke by gender and age on DALYs stroke in Indonesia. Indian J Forensic Med Toxicol 2020; 14(4):4457-63. [ Google Scholar]
- Jeong NJ, Park E, Del Pobil AP. Effects of behavioral risk factors and social-environmental factors on non-communicable diseases in South Korea: a national survey approach. Int J Environ Res Public Health 2021; 18(2):612. doi: 10.3390/ijerph18020612 [Crossref] [ Google Scholar]
- Azadnajafabad S, Mohammadi E, Aminorroaya A, Fattahi N, Rezaei S, Haghshenas R, et al. Non-communicable diseases’ risk factors in Iran; a review of the present status and action plans. J Diabetes Metab Disord. 2021:1-9. 10.1007/s40200-020-00709-8.