Avicenna J Environ Health Eng. 10(1):32-37.
doi: 10.34172/ajehe.2023.5259
Original Article
The Removal of Methylene Blue from Aqueous Solutions Using Zinc Oxide Nanoparticles With Hydrogen Peroxide
Somayeh Bagheri 1  , Fahimeh Moghadam 2, 3, *
, Fahimeh Moghadam 2, 3, *  , Hassn Mohammadi 4, Saeid Rigi 4
, Hassn Mohammadi 4, Saeid Rigi 4
Author information:
1Department of Biostatistics, School of Health, Zabol University of Medical Sciences, Zabol, Iran
2Student Research Committee, Department of Environmental Health Engineering, School of Health, Mashhad University of Medical Sciences, Mashhad, Iran
3Department of Environmental Health Engineering, School of Health, Zabol University of Medical Sciences, Zabol, Iran
4Student Research Committee, Department of Environmental Health, School of Health, Zabol University of Medical Sciences, Zabol, Iran
Abstract
Industrial effluents produce vast amounts of pollutants and account for 20% of industrial wastewater annually. Methylene blue (MB) is one of the most widely used dyes in the medical, pharmaceutical, and textile industries. However, it is toxic to living organisms, and even a short-time exposure to it can be potentially harmful. Therefore, the purpose of this study was to investigate the efficiency of zinc oxide nanoparticles in removing MB from aqueous solutions in the presence of hydrogen peroxide. The effects of various parameters such as pH (3-10), ZnO nanoparticle dose (0.01-0.08 g/L), reaction time (5-50 minutes), initial concentration of MB (20-200 mg/L), and the hydrogen peroxide concentration (0.5-5 mg/L) were studied. The wavelength of maximum absorption (λmax) was 665 nm. The optimal pH value was 5, zinc oxide nanoparticle dose was 0.05 g/L, the initial concentration of MB was 40 mg/L, the concentration of hydrogen peroxide was 2 mg/L, and the contact time was 20 minutes. The efficiency of MB removal was 97.99%. The results showed that zinc oxide nanoparticles in the presence of hydrogen peroxide could remove the MB from aqueous solutions with high efficiency.
Keywords: Methylene blue, Zinc oxide nanoparticles, Hydrogen peroxide, Aqueous solutions,
Copyright and License Information
© 2023 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Bagheri S, Moghadam F, Mohammadi H, Rigi S. The removal of methylene blue from aqueous solutions using zinc oxide nanoparticles with hydrogen peroxide. Avicenna J Environ Health Eng. 2023; 10(1):32-37. doi:10.34172/ ajehe.2023.5259
1. Introduction
Environmental pollution is a serious global issue (1). Today, dye-containing effluents are among the biggest challenges of significant industries such as textiles, pulp and paper, leather and plastics, and dyeing. Since these industries consume considerable amounts of water, they introduce significant amounts of dye-containing effluents into the environment (2-4). Methylene blue (MB) is the most common dye used in dyeing cotton, wool, and silk (5,6). Its inhalation can cause respiratory distress, and direct exposure to it leads to local burns, nausea, vomiting, increased sweating, mental disorders, and methemoglobinemia (7,8). The removal of dye from industrial wastewaters is carried out by various methods such as coagulation and flocculation, biological treatment, chemical oxidation, electrochemical treatment, ion exchange, and adsorption processes (5,9,10). According to a previous study, the efficiency of MB adsorption using activated carbon was up to 91%; however, activation and reduction of carbon are not cost-effective (11). Various biological methods have been applied to remove the dye; however, they were inefficient due to their low biodegradability (12).
Magnetic nanoparticles as adsorbents have been investigated recently (13-15). These nanoparticles are particles with dimensions up to 100 nm with magnetic properties (16). They have unique physical and chemical properties (17). Therefore, this study aimed to apply cost-effective zinc oxide nanoparticles with hydrogen peroxide due to their high absorption and environmentally friendly properties. They also have no secondary pollution and are very safe for this application. To establish the thermodynamic parameters, we also measured the pH, the concentration of MB, and the adsorbent solution. We also studied reaction time, oxidized nanoparticles dose, initial concentration of MB, concentration of H2O2, and contact time to find the optimal parameters for dye removal.
2. Materials and Methods
The adsorbent used in this research was zinc oxide nanoparticle (ZnO) which was obtained from Pasteur Institute in Tehran. The materials needed in this study include hydrochloric acid (HCl), sodium hydroxide (NaOH) for adjusting pH, zinc oxide nanoparticles, hydrogen peroxide, and MB dye. All test solutions were prepared with distilled water.
2.1. Methods
Distilled water was used to prepare all test solutions. The residual concentration (C1) of MB dye was also measured using a spectrophotometer (Cecil 1021) at a wavelength of 665 nm. To optimize the factors affecting the process, different values were tested as follows: pH (3,5,7,9-10-12), dose of zinc oxide nanoparticles (0.01, 0.02, 0.03, 0.04, 0.05, 0.06, and 0.08 g/L), initial concentration of MB (20, 30, 40, 50, 100, 150, and 200 mg/L), concentration of hydrogen peroxide (0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, and 5 mg/L), and contact time (5, 10, 15, 20, 30, 40, and 50 minutes). To optimize every parameter, we kept the others unchanged. The experiments had two replicates (18). First, the stock solution of MB was prepared. Then, different concentrations were prepared from the stock solution. A standard curve was prepared by reading the absorption using a spectrophotometer. In the first step, we studied the optimum dye concentration through different color concentrations at a fixed pH of 5, contact time of 20 minutes, nanoparticle concertation of 0.05 g/L, and H2O2 concentration of 2 mg/L. Therefore, we established the optimal concentration of the dye. The optimal pH was calculated in the second step by adjusting the pH with 0.1 M hydrochloric acid and 0.1 M sodium hydroxide. Next, the effluent concentration of MB for each sample was measured. In this step, a nanoparticle concentration of 0.05 g/L, MB concentration of 40 mg/L, contact time of 20 minutes, and hydrogen peroxide concentration of 2 mg/L were considered. In the third step, we studied different concentrations of ZnO nanoparticles. According to the previous steps, an MB concentration of 40 mg/L, a pH value of 5, a contact time time of 20 minutes, and an H2O2 concentration of 2 mg/L were considered and the results were measured with a spectrophotometer. In the fourth step, the amount of H2o2 was calculated. In this step, a certain amount of parameters was considered as optimal. The amount of H2O2 was determined at an MB concentration of 40 mg/L, a pH value of 5, a contact time of 20 minutes, and a nanoparticle concentration of 0.05 g/L. In the fifth step, the contact time was studied at 6 different time points (5-50 minutes), at the optimum dye concentration of 40 mg/L, and optimum pH value of 5, as well as the optimum H2O2 concentration of 2 mg/L. The corresponding equations are as follows:
C0 = Inlet concentration of MB dye
C1 = MB dye output concentration
R = Percentage of MB dye removal efficiency
3. Results and Discussion
3.1. Effect of Initial MB Concentration on the Removal Efficiency
The first step in the optimization of the MB removal was to determine the optimal initial concentration of MB. The results presented in Fig. 1 showed that the highest removal efficiency of MB was observed at the initial concentration of 40 mg/L. According to this figure, by increasing concentration from 20 to 40 mg/L, the removal efficiency of MB was increased, but at concentrations above 40 mg/L, the removal efficiency decreased. Therefore, the initial optimum concentration of MB in this process was determined to be 40 mg/L. The effect of the initial concentration of MB on the adsorbent efficiency was shown in Fig. 1. The relationship between the equilibrium concentration of MB after adsorption and the adsorption rate on the adsorbent surface was also evaluated. The figure shows that increasing the initial concentration of MB from 20 to 50 mg/L increased the removal rate, and then the value remained constant. The highest removal rate obtained at an MB concentration of 50 mg/L was 94.83%. The reason is that the adsorbent has specific and limited adsorption sites. More adsorption sites are accessible at lower concentrations on the adsorbent surface, leading to rapid adsorption and higher removal efficiency. However, at higher concentrations, as the adsorbate on the adsorbent surface increases, the adsorption sites are occupied rapidly, and the removal efficiency decreases. Faraji et al and Akbari et al also reported similar results (19,20). It is suggested that the increasing adsorption capacity of adsorbents with increasing the initial concentration of MB is due to the increased probability of collision and contact between the adsorbent and the adsorbate (20).
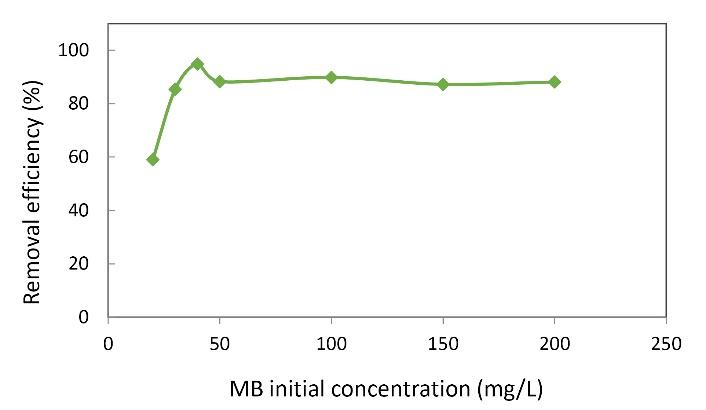
Fig. 1.
Effect of Different Concentrations of Methylene Blue on Removal Efficiency. (Nanoparticle dose: 0.05 g/L, pH: 5, contact time: 20 minutes, H2O2 concentration: 2 mg/L)
.
Effect of Different Concentrations of Methylene Blue on Removal Efficiency. (Nanoparticle dose: 0.05 g/L, pH: 5, contact time: 20 minutes, H2O2 concentration: 2 mg/L)
3.2. Effect of pH on MB Removal Efficiency
The second step in the optimization of the MB removal process was to determine the optimal pH. Examination of the results of the following diagram in determining the optimal pH of MB removal showed that the highest removal efficiency of MB occurred at pH = 5, which is equal to 94.83%. With increasing pH from 3 to 5, the removal efficiency increased, but with increasing the pH from 6 to 10, the removal efficiency decreased. Fig. 2 shows that the removal efficiency was raised by increasing pH from 3 to 5 and decreased at pH values above 5. It seems that this increase occurred due to dye degradation at acidic pH and also formation of a smaller amount of species with low reactivity, such as per hydroxyl radical radicals (OOH°) compared to hydroxyl radicals (21). However, by increasing pH, additional OH- ions may form ferric hydroxide complexes, thereby reducing the hydroxyl radicals and the rate of degradability (22). In addition, the higher pH of the solution may result in large amounts of hydroxyl radical scavenging agents (e.g., carbonates and bicarbonates). They are formed by the mineralization of organic matter and finally decrease of the OH- concentration (23). The results are consistent with those of the study by Rahdar et al on the removal of ciprofloxacin from contaminated water by nickel nanoparticles (24).
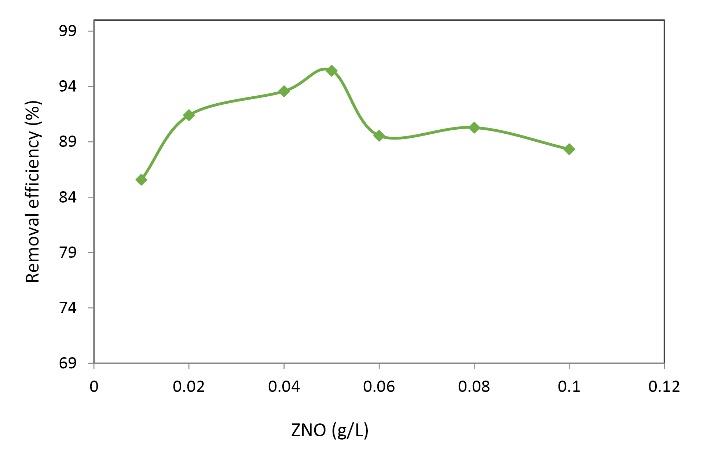
Fig. 2.
Effect of Different pH Values on the Removal of Methylene Blue. (Nanoparticle dose: 0.05 g/L, initial MB concentration: 40 mg/L, contact time: 20 minutes, H2O2 concentration: 2 mg/L)
.
Effect of Different pH Values on the Removal of Methylene Blue. (Nanoparticle dose: 0.05 g/L, initial MB concentration: 40 mg/L, contact time: 20 minutes, H2O2 concentration: 2 mg/L)
3.3. The Effect of Zinc Oxide Nanoparticle Concentration on the Removal Efficiency
The third step in optimizing the parameters in the MB removal process was to optimize the dose of zinc oxide nanoparticles. Fig. 3 shows the effect of zinc oxide nanoparticle concentration on MB removal efficiency. The results of this diagram show that in the process of removing MB at an optimal pH of 5, a constant contact time of 20 minutes, and a concentration of 40 mg/L MB, by increasing nanoparticle dose from 0.01 to 0.05 g/L, an increase in MB removal efficiency was observed from 85.59 to 95.43%. However, as it is clear from the figure, by increasing the absorbent dose from 0.05 g/L, the removal efficiency of garlic was decreased. The optimum dose of nanoparticles was determined to be 0.05 g/L. The optimum dose of nanoparticles was determined to be 0.05 g/L. Fig. 3 shows the effect of nanoparticle dose on MB removal rate. It can be observed that increasing the amount of adsorbent dose elevated the removal efficiency. Therefore, increasing the nanoparticle dose from 0.01 to 0.05 enhances the removal efficiency. The highest removal rate of 95.43% was seen at a nanoparticle dose of 0.05. The figure also showed that the removal rate decreased at concentrations higher than 0.05 g/L. The growing rate of MB adsorption by increasing the adsorbent may be due to extended active and effective surface area in adsorption. These trends were also reported by Ong et al (25) and have also been confirmed by Sulak et al (26).
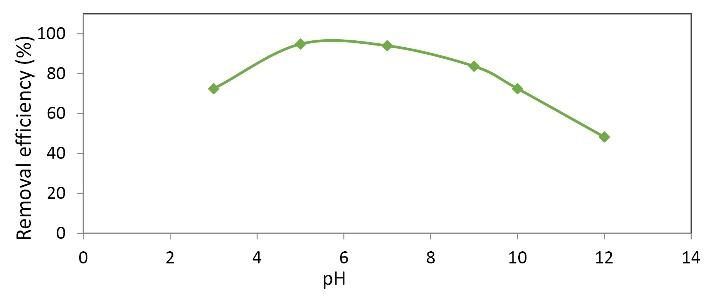
Fig. 3.
Effect of Zinc Oxide Nanoparticle Dose on the Removal of MB. (Initial MB concentration: 40 mg/L, pH: 5, contact time: 20 minutes, H2O2 concentration: 2 mg/L)
.
Effect of Zinc Oxide Nanoparticle Dose on the Removal of MB. (Initial MB concentration: 40 mg/L, pH: 5, contact time: 20 minutes, H2O2 concentration: 2 mg/L)
3.4. Effect of Hydrogen Peroxide Concentration on the Removal Efficiency
This step was related to the optimization of the dose of hydrogen peroxide in the MB removal process.Fig. 4 shows the effect of hydrogen peroxide on the MB removal efficiency. The figure illustrated how different values (0.5 to 2 mg) increased the removal rate and then decreased it. The results presented in the figure shows that in the process of removing MB, by increasing the dose of hydrogen peroxide from 0.5 to 2 mg/L, the removal efficiency of MB was increased from 83.64 to 95.41%. However, as can be seen from the graph, by increasing the dose of hydrogen peroxide from 2 mg/L, the removal efficiency was decreased, indicating that at the dose of 5 mg/L, the removal efficiency reached its lowest level, which was 70.43%. Besides, higher concentrations reduced the removal of Reactive Blue 19 dye because H2O2 alone could not oxidize organic compounds which are resistant to biodegradation, and the presence of ferrous ions led to the formation of a small amount of hydroxyl radicals (27). These findings are consistent with the study of Barbusiński et al on the removal of Acid Red 18 by Fenton reagent in the presence of iron powder (28). On the other hand, in the presence of hydrogen peroxide, the amount of H2O2 was decreased by increasing pH. This phenomenon is due to the instability of peroxide in alkaline conditions, which leads to the decomposition of peroxide into water and oxygen and reduces the efficiency of the process (29,30). It was observed that the color removal percentage decreased with the increase of peroxide concentration. This phenomenon can be explained by the fact that with the higher concentration of H2o2, the hydroxyl radicals produced in the following reaction react with hydrogen peroxide, and the peroxide itself acts as an OH° radical absorbent. Finally, it leads to the production of hydroxyl radicals (OH°2), which have a lower oxidizing power compared to OH° radicals (30-32).
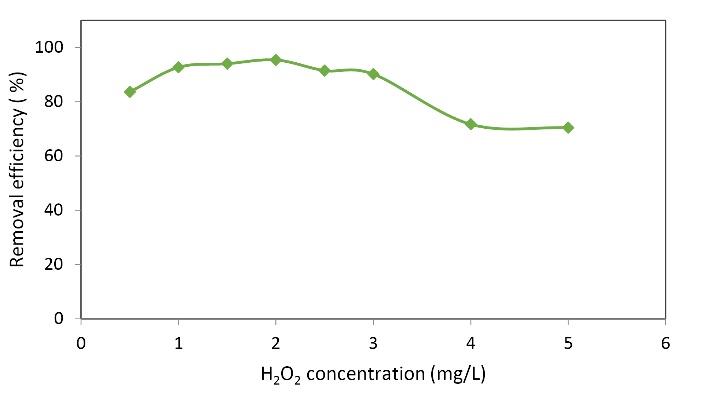
Fig. 4.
Effect of H2O2 Concentration on the Removal of MB. (Nanoparticle dosage: 0.05 g/L, initial MB concentration: 40 mg/L, pH: 5, contact time: 20 minutes)
.
Effect of H2O2 Concentration on the Removal of MB. (Nanoparticle dosage: 0.05 g/L, initial MB concentration: 40 mg/L, pH: 5, contact time: 20 minutes)
3.5. Effect of Contact Time on the Removal Efficiency
The last step in the process was to evaluate the effect of different contact times. As seen in Fig. 5, increasing the contact time from a certain limit led to a decrease in removal efficiency. In other words, the maximum removal efficiency usually occurred in the first 20 minutes of the reaction. Given that time is a factor that has a significant impact on the process in terms of cost and energy, a contact time of 20 minutes was chosen as the optimal contact time. Fig. 5 represents the effects of contact time on the rate of removal by oxidized nanoparticles. It shows that the efficiency was improved by increasing contact time. Therefore, in the first 20 minutes with a 95% removal rate, the removal speed was reduced with a smaller slope. In this way, at 40 minutes, the removal efficiency was only 60%. In the early stages of adsorption, many vacant surface sites were accessible; however, they were occupied over time and could not absorb pollutants any more. This can be due to the restraining forces between the adsorbed molecules on the surface of the solid adsorbent and the liquid mass. Similar results have been reported by Cengiz and Cavas (33). These results are also consistent with the findings of a study by Moghadam et al (34). Moreover, the results of various studies on the removal of MB by nanoparticles are given in Table 1.
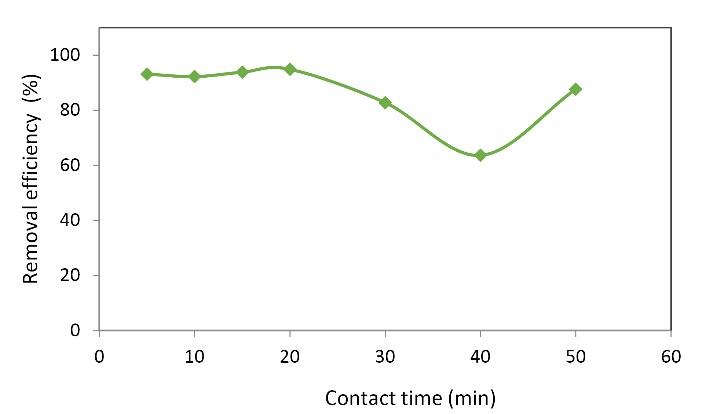
Fig. 5.
Effect of Contact Time on the Removal of MB. (Nanoparticle dosage: 0.05 g/L, initial MB concentration: 40 mg/L, pH: 5, H2O2 concentration: 2 g/L)
.
Effect of Contact Time on the Removal of MB. (Nanoparticle dosage: 0.05 g/L, initial MB concentration: 40 mg/L, pH: 5, H2O2 concentration: 2 g/L)
Table 1.
The Results of Different Studies on the Removal of Methylene Blue by Nanoparticles
|
Study
|
Nanoparticle
|
Removal (%)
|
|
Zaman et al (35)
|
Zinc oxide nanoparticles contaminated with lead |
90% |
|
Ansari et al (36)
|
Magnesium oxide nanoparticles |
95% |
|
Darvishi Cheshmeh Soltani et al (37)
|
Milk vetch wood |
80% |
|
Razavi (38)
|
Titanium oxide nanoparticles |
70% |
|
Tavakoli et al (39)
|
Nanocomposite |
70-10% |
|
Alomar et al (40)
|
Bi2O3-SrO-FeO@SiO2 nanocomposite |
> 80% |
4. Conclusion
Our results suggested that 0.05 g/L of zinc oxide nanoparticles and 2 mg/L of hydrogen peroxide in 20 minutes could remove the MB from the water with a rate of approximately 97.99%. Zinc oxide nanoparticles are a very potent adsorbent and, at the same time, cost-effective for the removal of various pollutants from water. The application of hydrogen peroxide in this process has also improved the removal efficiency and decreased the required contact time. The removal efficiency of 98% was obtained for the MB in the optimum conditions.
Acknowledgments
This research project entitled “Methylene blue removal from aqueous solutions by zinc oxide nanoparticles with hydrogen peroxide” was approved by Zabol University of Medical Sciences (IR.ZBMU.REC.1398.048). The authors are thankful to the Research Deputy of the Zabol University of Medical Sciences for meeting the financial and credit requirements of this thesis.
Authors’ Contribution
Conceptualization: Fahimeh Moghaddam.
Data curation: Fahimeh Moghaddam, Hassan Mohammadi, Saeid Rigi.
Formal analysis: Somayeh Bagheri.
Investigation: Hassan Mohammadi, Saeid Rigi.
Methodology: Fahimeh Moghaddam.
Project administration: Fahimeh Moghaddam,Somayeh Bagheri.
Resources: Fahimeh Moghaddam,Somayeh Bagheri.
Software: Fahimeh Moghaddam.
Supervision: Fahimeh Moghaddam,Somayeh Bagheri.
Validation: Fahimeh Moghaddam,Somayeh Bagheri.
Writing–original draft: Fahimeh Moghaddam,Somayeh Bagheri.
Writing–review & editing: Fahimeh Moghaddam.
Competing Interests
The authors declare that they have no conflict of interest.
References
- Paul J, Rawat KP, Sarma KS, Sabharwal S. Decoloration and degradation of Reactive Red-120 dye by electron beam irradiation in aqueous solution. Appl Radiat Isot 2011; 69(7):982-7. doi: 10.1016/j.apradiso.2011.03.009 [Crossref] [ Google Scholar]
- Öden MK, Özdemir C. Removal of methylene blue dye from aqueous solution using natural boron ore and leach waste material: adsorption optimization criteria. Int J Curr Res Acad Rev 2014; 1:66-71. [ Google Scholar]
- Mondal S. Methods of dye removal from dye house effluent—an overview. Environ Eng Sci 2008; 25(3):383-96. doi: 10.1089/ees.2007.0049 [Crossref] [ Google Scholar]
- García-Montaño J, Torrades F, Perez-Estrada LA, Oller I, Malato S, Maldonado MI. Degradation pathways of the commercial reactive azo dye Procion Red H-E7B under solar-assisted photo-Fenton reaction. Environ Sci Technol 2008; 42(17):6663-70. doi: 10.1021/es800536d [Crossref] [ Google Scholar]
- dos Santos AB, Cervantes FJ, van Lier JB. Review paper on current technologies for decolourisation of textile wastewaters: perspectives for anaerobic biotechnology. Bioresour Technol 2007; 98(12):2369-85. doi: 10.1016/j.biortech.2006.11.013 [Crossref] [ Google Scholar]
- Attia AA, Girgis BS, Fathy NA. Removal of methylene blue by carbons derived from peach stones by H3PO4 activation: batch and column studies. Dyes Pigm 2008; 76(1):282-9. doi: 10.1016/j.dyepig.2006.08.039 [Crossref] [ Google Scholar]
- Ponnusami V, Madhuram R, Krithika V, Srivastava SN. Effects of process variables on kinetics of methylene blue sorption onto untreated guava (Psidium guajava) leaf powder: statistical analysis. Chem Eng J 2008; 140(1):609-13. doi: 10.1016/j.cej.2007.11.003 [Crossref] [ Google Scholar]
- Rafatullah M, Sulaiman O, Hashim R, Ahmad A. Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 2010; 177(1-3):70-80. doi: 10.1016/j.jhazmat.2009.12.047 [Crossref] [ Google Scholar]
- Slokar YM, Majcen Le Marechal A. Methods of decoloration of textile wastewaters. Dyes Pigm 1998; 37(4):335-56. doi: 10.1016/s0143-7208(97)00075-2 [Crossref] [ Google Scholar]
- Karagozoglu B, Tasdemir M, Demirbas E, Kobya M. The adsorption of basic dye (Astrazon Blue FGRL) from aqueous solutions onto sepiolite, fly ash and apricot shell activated carbon: kinetic and equilibrium studies. J Hazard Mater 2007; 147(1-2):297-306. doi: 10.1016/j.jhazmat.2007.01.003 [Crossref] [ Google Scholar]
- Poormand H, Leili M, Khazaei M. Adsorption of methylene blue from aqueous solutions using water treatment sludge modified with sodium alginate as a low cost adsorbent. Water Sci Technol 2017; 75(2):281-95. doi: 10.2166/wst.2016.510 [Crossref] [ Google Scholar]
- Almeida CA, Debacher NA, Downs AJ, Cottet L, Mello CA. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J Colloid Interface Sci 2009; 332(1):46-53. doi: 10.1016/j.jcis.2008.12.012 [Crossref] [ Google Scholar]
- Lü K, Zhao G, Wang X. A brief review of graphene-based material synthesis and its application in environmental pollution management. Chin Sci Bull 2012; 57(11):1223-34. doi: 10.1007/s11434-012-4986-5 [Crossref] [ Google Scholar]
- Shariati S, Faraji M, Yamini Y, Rajabi AA. Fe3O4 magnetic nanoparticles modified with sodium dodecyl sulfate for removal of safranin O dye from aqueous solutions. Desalination 2011; 270(1-3):160-5. doi: 10.1016/j.desal.2010.11.040 [Crossref] [ Google Scholar]
- Zhou L, Gao C, Xu W. Magnetic dendritic materials for highly efficient adsorption of dyes and drugs. ACS Appl Mater Interfaces 2010; 2(5):1483-91. doi: 10.1021/am100114f [Crossref] [ Google Scholar]
- Mohapatra M, Anand S. Synthesis and applications of nano-structured iron oxides/hydroxides–a review. Int J Eng Sci Technol 2010; 2(8):127-46. doi: 10.4314/ijest.v2i8.63846 [Crossref] [ Google Scholar]
- Leslie-Pelecky DL, Rieke RD. Magnetic properties of nanostructured materials. Chem Mater 1996; 8(8):1770-83. doi: 10.1021/cm960077f [Crossref] [ Google Scholar]
- Moghadam F, Nori Kohbanan N. Removal of Reactive Blue 19 dye using Fenton from aqueous solution. Avicenna J Environ Health Eng 2018; 5(1):50-5. doi: 10.15171/ajehe.2018.07 [Crossref] [ Google Scholar]
- Faraji H, Naseri S, Amouei A, Mohammadi F, Soheilarezomand H, Mahvi AH. Survey electrocoagulation process in removal of Acid Blue 113 dye from aqueous solutions. J Environ Health Eng 2015; 2(2):98-107. doi: 10.18869/acadpub.jehe.2.2.98.[Persian] [Crossref] [ Google Scholar]
- Akbari A, Desclaux S, Rouch JC, Aptel P, Remigy JC. New UV-photografted nanofiltration membranes for the treatment of colored textile dye effluents. J Memb Sci 2006; 286(1-2):342-50. doi: 10.1016/j.memsci.2006.10.024 [Crossref] [ Google Scholar]
- Gan PP, Li SFY. Efficient removal of Rhodamine B using a rice hull-based silica supported iron catalyst by Fenton-like process. Chem Eng J 2013; 229:351-63. doi: 10.1016/j.cej.2013.06.020 [Crossref] [ Google Scholar]
- Nidheesh PV, Gandhimathi R, Sanjini NS. NaHCO3 enhanced Rhodamine B removal from aqueous solution by graphite–graphite electro Fenton system. Sep Purif Technol 2014; 132:568-76. doi: 10.1016/j.seppur.2014.06.009 [Crossref] [ Google Scholar]
- Cuiping B, Xianfeng X, Wenqi G, Dexin F, Mo X, Zhongxue G. Removal of Rhodamine B by ozone-based advanced oxidation process. Desalination 2011; 278(1-3):84-90. doi: 10.1016/j.desal.2011.05.009 [Crossref] [ Google Scholar]
- Rahdar S, Rahdar A, Igwegbe CA, Moghaddam F, Ahmadi S. Synthesis and physical characterization of nickel oxide nanoparticles and its application study in the removal of ciprofloxacin from contaminated water by adsorption: equilibrium and kinetic studies. Desalin Water Treat 2019; 141:386-93. doi: 10.5004/dwt.2019.23473 [Crossref] [ Google Scholar]
- Ong ST, Lee CK, Zainal Z. Removal of basic and reactive dyes using ethylenediamine modified rice hull. Bioresour Technol 2007; 98(15):2792-9. doi: 10.1016/j.biortech.2006.05.011 [Crossref] [ Google Scholar]
- Sulak MT, Demirbas E, Kobya M. Removal of Astrazon Yellow 7GL from aqueous solutions by adsorption onto wheat bran. Bioresour Technol 2007; 98(13):2590-8. doi: 10.1016/j.biortech.2006.09.010 [Crossref] [ Google Scholar]
- Kashefialasl M, Khosravi M, Marandi R, Seyyedi K. Treatment of dye solution containing colored index Acid Yellow 36 by electrocoagulation using iron electrodes. Int J Environ Sci Technol 2006; 2(4):365-71. [ Google Scholar]
- Barbusiński K. The modified Fenton process for decolorization of dye wastewater. Pol J Environ Stud 2005; 14(3):281-5. [ Google Scholar]
- Haber F, Weiss J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc R Soc Lond A Math Phys Sci 1934; 147(861):332-51. doi: 10.1098/rspa.1934.0221 [Crossref] [ Google Scholar]
- Pignatello JJ, Oliveros E, MacKay A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 2006; 36(1):1-84. doi: 10.1080/10643380500326564 [Crossref] [ Google Scholar]
- Jamshidi N, Torabian A, Azimi A, Nabi Bidhendi G, Jafarzadeh MT. Investigation of phenol removal in aqueous solutions using advanced photochemical oxidation (APO). J Water Wastewater 2009;20(4):24-9. [Persian].
- Zhang H, Zhang D, Zhou J. Removal of COD from landfill leachate by electro-Fenton method. J Hazard Mater 2006; 135(1-3):106-11. doi: 10.1016/j.jhazmat.2005.11.025 [Crossref] [ Google Scholar]
- Cengiz S, Cavas L. Removal of methylene blue by invasive marine seaweed: Caulerpa racemosavar cylindracea. Bioresour Technol 2008; 99(7):2357-63. doi: 10.1016/j.biortech.2007.05.011 [Crossref] [ Google Scholar]
- Moghadam F, Bagheri S, Khammar M. Evaluation of chitosan adsorbent efficiency in nitrate removal from aqueous solutions and the effect of parameters on adsorption process. Int J Innov Sci Eng Technol 2018; 5(5):83-6. [ Google Scholar]
- Zaman L, Yousefi R, Niayi Far M. Removal of methylene blue dye with the help of zinc oxide nanoparticles contaminated with lead. Proceedings of the 6th Water, Effluent and Waste Conference; 2016.
- Ansari R, Mohammadpour Tasih A, Rasooly Garmaroody E, Kermanian H. Removal of methylene blue by sawdust coated with magnesium oxide nanoparticles. Appl Chem 2018; 13(46):219-38. doi: 10.22075/chem.2017.10419.1014.[Persian] [Crossref] [ Google Scholar]
- Darvishi Cheshmeh Soltani R, Noorimotlagh Z, Shahryar S, Nourmoradi H, Rahmati Z, Nazari S. Application of milk vetch wood as adsorbent of methylene blue dye from aqueous solution. J Ilam Univ Med Sci 2016; 24(4):41-52. doi: 10.18869/acadpub.sjimu.24.4.41.[Persian] [Crossref] [ Google Scholar]
- Razavi SA. Investigation of methylene blue removal using titanium oxide nanoparticles from aqueous solutions. Proceedings of the 6th Water, Effluent and Waste Conference; 2016.
- Tavakoli F, Bakhtiyari F, Dare Zereshki E. Removal of methylene blue dye from solution by nanocomposite. Iran’s National Environmental Research Conference; 2016.
- Alomar TS, AlMasoud N, Sharma G, Alothman ZA, Naushad M. Incorporation of trimetallic nanoparticles to the SiO2 matrix for the removal of methylene blue dye from aqueous medium. J Mol Liq 2021; 336:116274. doi: 10.1016/j.molliq.2021.116274 [Crossref] [ Google Scholar]