Avicenna J Environ Health Eng. 9(1):9-17.
doi: 10.34172/ajehe.2022.02
Original Article
Bulking Control in Complete-Mixed Activated Sludge Process Using Combination of Metallic Coagulants and Static Magnetic Fields
Ghorban Asgari 1  , Abdolmotaleb Seid-Mohammadi 1, Ramin Khoshniyat 1, *
, Abdolmotaleb Seid-Mohammadi 1, Ramin Khoshniyat 1, *  , Esmaeil Ghahramani 1, Hana Shabrandi 2
, Esmaeil Ghahramani 1, Hana Shabrandi 2
Author information:
1Social Determinants of Health Research Center (SDHRC), Faculty of Public Health, Department of Environmental Health Engineering, Hamadan University of Medical Sciences, Hamadan, Iran
2Supervisor of Sanandaj Wastewater Treatment Laboratory, Kurdistan Water and Wastewater Engineering Company, Sanandaj, Iran
Abstract
Metallic coagulants have been used for more coagulation and flocculation of flocs in many wastewater treatment plants (WWTPs) in all parts of the world. The integration of different methods to improve the wastewater treatment process has been considered in recent years. In this case-control study, the effects of four main coagulants (ferric chloride, ferric sulfide, alum, and poly-aluminum chloride) on sludge volume index (SVI) with and without exposure of static magnetic fields (SMFs) have been investigated. Both methods significantly reduced SVI (mL/g), but the combination of SMFs and coagulants was more effective. Ferric chloride could control bulking or reduce SVI to less than 150 mL/g at concentrations of 0.0625 to 2 g/L when the SMFs intensity of 15 mT was used. The control of bulking in other coagulants happened when SMFs were added to coagulants at 0.0625-0.125 g/L concentration of coagulants (P<0.05). With the application of SMFs, the highest reduction of SVI belonged to ferric sulfide (43.60%), followed by ferric chloride (18.40%), poly-aluminum chloride (PACl) (20.19%), and alum (19.80%). Without the application of SMFs, the highest reduction of SVI belonged to ferric chloride (38.36%), followed by alum (34.94%), PACl (25.43%), and ferric sulfide (6.69%).
Keywords: Wastewater treatment, Magnetic fields, Coagulants
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Asgari G, Seid-Mohammadi A, Khoshniyat R, Ghahramani E, Shabrandi H. Bulking control in completemixed activated sludge process using combination of metallic coagulants and static magnetic fields. Avicenna J Environ Health Eng. 2022; 9(1):9-17. doi:10.34172/ajehe.2022.02
Introduction
Activated sludge (AS) process is the most widely used biological method for treatment of municipal and industrial wastewater (1). AS has been widely used to remove nutrients and toxic materials for more than a century (2). AS has been used for the treatment of industrial and domestic wastewater by considering the main role of the community of bacteria and other microorganisms such as protozoa and metazoan (3). AS process has many benefits but, there are some problems with the application of AS such as the generation of a huge volume of sludge and the need for reuse and reduction of sludge. Economic and environmental conditions of sludge management are other aspects of this kind of aerobic wastewater treatment (4). It was stated that approximately 45% of the costs of wastewater treatment plants (WWTPs) are attributed to sludge management (5).
Management of sludge in WWTPs is the key factor to reduce the operational cost. In the course of sludge management, the increase in sludge density has a critical effect on the cost of handling and transportation of sludge from WWTPs to the site of disposal (6). Therefore, in order to reduce the operational cost, each process that could properly separate the biomass from treated wastewater is considered a well-known factor that affects the cost-benefit condition. The use of gravity sedimentation is an economical method for separation of solids (flocs) from the liquid phase in some wastewater treatment processes (3). Nowadays, use of mechanical and chemical methods to higher augmentation of flocs size and higher separation of solids from a liquid phase, have become more popular (7).
The application of metallic ions in WWTPs by creating a cross-link between flocs was reported to have a key role in the aggregation of sludge (8). Aluminum and iron salts are two main inorganic coagulants that are used in water and wastewater treatment processes for substantial reduction of sludge volume (9). Aluminum could increase the compactness of sludge, sludge size, and surface area of wastewater when it is used as a coagulant (10). Ferric chloride is used for improving AS, in terms of the neutralization of negative charge, sedimentation of flocs by surface modification of the matrix in sludge, dewatering of sludge, and improvement of the settling velocity of flocs (11,12).
In order to improve the efficiency of the sludge coagulation and flocculation process, some researchers proposed the application of chemical materials combined with other methods (13). However, few studies have been done on the use of hybrid methods for sludge conditioning (14,15).
Some of the most important parameters for selection of a new method to improve WWTPs include low cost, safety for the environment, the possibility of the reuse of materials, and efficiency (16). One of the methods that has been considered by many researchers is the application of static magnetic fields (SMFs) for water and wastewater treatment process. SMFs are magnetic fields that do not vary in intensity over time and have a frequency of 0 Hz (17,18). The application of SMFs for treatment of wastewater could increase the physical properties of wastewater components and, improve the separation of solids from liquid phase. Coagulation of colloidal particles is the reason for this phenomenon. Additionally, the increase in the activity of bacteria in wastewater is the other benefit of this method (19).
In this case-control study, the effects of the use of SMFs and four main coagulants (there are the most commonly used coagulants in water and wastewater treatment) on the sludge volume index (SVI) of mixed liquor suspended solids (MLSS) of complete-mixed AS process were investigated.
2. Materials and Methods
2.1. Coagulants
Ferric chloride, ferric sulfide, aluminum sulfide (alum), and poly-aluminum chloride (PACl) are four metallic coagulants that were used in this study (Fig. 1). The concentrations of these coagulants used in our study were 0.0625, 0.125, 0.25, 0.5, 1, and 2 g/L. The high concentration of coagulants was selected to see the change of pH in the samples and find the turning point of pH changes.

Figure 1.
Four Metallic Coagulants Used in This Study
.
Four Metallic Coagulants Used in This Study
2.2. Mixed Liquor Suspended Solids
All samples (the cases and control) of MLSS (mg/L) were obtained from the effluent aeration basin of Sanandaj WWTPs (the capital city of Kurdistan that is located in the west of Iran) daily. The complete-mixed activated sludge (CMAS) process was used in this plant for the treatment of wastewater. After sampling and transferring the samples to the laboratory, they were stored at 4 °C to avoid any changes. All runs of the experiments were done in one day, so at the beginning of each run, the concentration of MLSS (mg/L) was measured. Each run of experiments was repeated three times to ensure the accuracy of the obtained results.
2.3. pH and Temperature
As the concentration of chemical materials in the solution is related to pH, pH has a key role in wastewater treatment process (20). In this study, the pH of MLSS (mg/L) from the main source (control sample) and the cases after adding coagulants and SMFs was measured by a digital pH meter (827 pH Lab Meter). Sedimentation of MLSS (mg/L) in the secondary basin of wastewater treatment can be affected by temperature (20). Therefore, the temperature of the cases and control samples was measured by digital thermometers (Testo 104 penetration thermometer) at the end of each run of the experiment. The measurement of pH and temperature was repeated three times for each run of the experiments.
2.4. SMFs Generation
Using solenoids that are connected to direct current (DC) is one of the ways used for generation of SMFs. To complete the electrical circuit, a light bulb should be added. Two main methods are proposed to calculate the SMFs. One of them is the use of Ampere’s Circuital Law and another is the use of Tesla-meter or Gauss meter (21). In this study, 15 mT was considered as the optimal intensity of SMFs (22,23). As in our study, the wires are wrapped around the galvanized iron cylinder (0.5 mm thickness, 10 cm diameter, and 25 cm height). This device intensifies the SMFs; therefore, using a Tesla-meter yields more realistic results than the equations. To produce this intensity of SMFs, 750 rounds of wires with 1 mm thickness were needed.
2.5. Jar Test
By the use of Jar-stirring device, 1000 mL of MLSS was mixed in 1-L beaker. Then, 0.0625 g of ferric chloride (the lowest concentration in our experiments) was added to the beaker and the solution was mixed at 200 rpm for 30 seconds and 50 rpm for 10 minutes (13). As the daily concentration of MLSS changed, we tried to do each step daily. In order to ensure accuracy, all experiments were repeated three times for all concentrations of coagulants. It is clear that these steps were repeated for other concentrations of other coagulants, too.
2.6. SVI Test
At the beginning of each run of the experiment, to calculate SVI (mL/g), based on the parts of 2710 D of Standard Methods for the Examination of Water and Wastewater, the amount of suspended solids (SS) was measured. Suspended solids from the effluent of aeration basin is called MLSS (mg/L) instead of SS. Then, the volume of settled sludge in the 1-L graduated cylinder after 30 minutes was estimated as SV30 (mL/L) and finally, SVI was calculated using the following formula (20):
(1)
SVI is the volume in mL occupied by 1 g of a suspension (mL/g).
Settled sludge volume or SV30 is the volume of sludge settled in a 1-L graduated cylinder after 30 minutes.
SS or MLSS in an aqueous sample (L) the mass (mg) of solids retained on a surface of glass-fiber filter.
2.7. Data Collection and Analysis
In this study, samples of case 1 were exposed to SMFs and coagulants were added to MLSS. Case 2 is the group that contained only coagulants and MLSS and the control group contained only MLSS without coagulants and exposure to SMFs. The concentrations selected for the four mentioned coagulants included 0.0625, 0.125, 0.25, 0.5, 1, and 2 g. Each run of the experiments included measurement of weight, pH, temperature, MLSS, and SV30. To ensure accuracy, each run was repeated three times. In this way, for each coagulant, 54 runs of experiments were designed for case 1, case 2, and control samples (18 runs for each). Data were analyzed using ANOVA and independent sample t test in SPSS version 21.
3. Results and Discussion
Ferric and aluminum salts are used as coagulants in the water and wastewater treatment process. They are highly recommended due to their compact structure, small size, high solid contents, and density (13).
The effect of MFs on biological systems and microorganisms depends on the kind of microorganism, time of exposure, temperature, and intensity of MFs (24). Some researchers reported that MFs could separate solids and colloidal particles from wastewater (1,19).
MFs could accelerate the coagulation of particles in sludge and, facilitate the dewatering of the sludge. MFs can change the structure of molecules in solution and reduce the amount of chemical materials in AS. Consequently, the consumption of materials decreases and the total cost of the process reduces significantly. Nevertheless, little information is available about the effect of MFs on AS (25).
There are three main mechanisms for coagulation and flocculation of water and wastewater: (1) sweep flocculation, (2) compression of double-layer, and (3) neutralization of charge (26,27). Double-layer compression is not suitable for wastewater treatment, so the two other methods are the most important processes (28). Additionally, in wastewater treatment process, the behavior of coagulants could be affected by the type of coagulants, pH, and characteristics of wastewater (29). Sludge particles are conditioned with inorganic coagulants. Charge neutralization happens in this process (30). Aluminum and ferric flocs have compact structures and high solid contents (13).
SV30, dose of coagulants, pH, and temperature are four main factors that have basic roles in measurement of SVI. In the flowing, the interaction between the concentration of four main coagulants with and without SMFs along with SV30, SVI, pH and temperature is presented. SV30 is the main factor for the measurement of SVI. The dose of coagulants is one of the most important parameters in the sludge conditioning process (31).
Regarding the application of coagulants for sludge conditioning, the efficiency of coagulants in dewatering and the economic situation are two main factors that need to be considered. Consumption of low doses of coagulants is always suggested because the consumption of high concentrations of coagulant materials can cause instability of the sludge flocs. This phenomenon is known as re-stabilization effect (32,33). The hydrolysis speed of chemical materials and precipitation of solids have key roles in solubility. They are related to the pH of a system. Ambient temperature has an effect on the determination of optimal doses of coagulants. In other words, by increasing the temperature of the solution, the dose of chemical materials decreases (33).
3.1. Effect of SMFs on SV30 in the Case and Control Samples
The effects of SMFs on SV30 in the samples of case 1 (containing coagulants placed in the MFs), case 2 samples (with only coagulants), and the control samples (without SMFs and coagulants) were illustrated in Fig. 2.
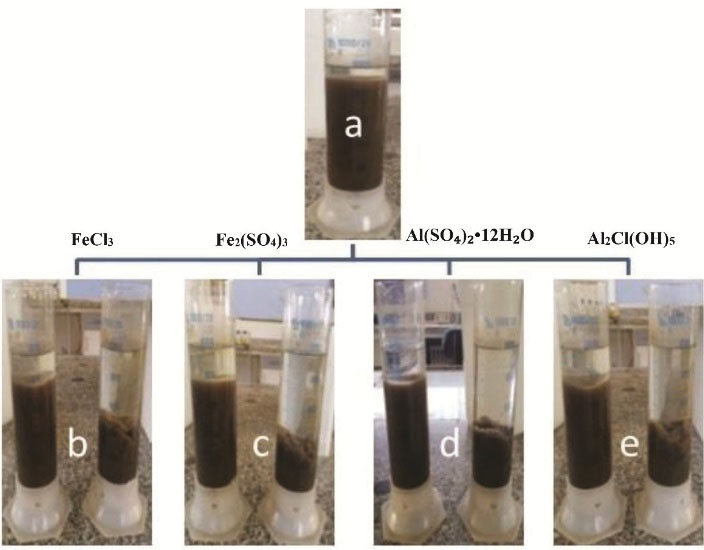
Figure 2.
SV30 in the Cases and Control Samples.
.
SV30 in the Cases and Control Samples.
The volume of SV30 in the control sample (a) is equal to 960 mL/L. SV30 in case 1 (on the right side) is equal to 430 (mL/L) and in the case 2 (on the left side) is 800 (mL/L) (Fig. 2b). Therefore, in this section of the study, the combination of SMFs and FeCl3 could reduce SV30 to 530 mL in case 1 compared to the control samples and to 370 mL compared to case 2.
The application of SMFs and Fe2(SO4)3 in the samples of case 1 reduced SV30 (360 mL/L) which is illustrated in Fig. 2b. This proportion is about 600 mL less than that of the control sample and 520 mL less than that of the case 2.
In this concentration levels of alum and PACl (0.0625 g), as shown in Fig. 2c and 2d, a reduction of 420 mL was occurred in SV30 in both of the coagulants compared to the control sample. This value for case 1 is 500 mL for alum when compared with case 2, and it is equal to 440 mL for PACl when compared with case 1.
It is necessary to mention that sedimentation of sludge in the graduated cylinders in which SMFs were present at the surface of sludge was rough and, in case 2 and the control samples were smooth.
Zieliński et al reported the effect of the MF intensity of 1.6 mT on coagulation and flocculation of SV30 using iron and aluminum in 2018. Four doses (0.05, 0.1, 0.2, and 0.4 g/dm3) of metallic coagulants were applied to bioreactors. They reported that unlike aluminum-based coagulant, the iron-based coagulant had no effect on SV30. The decrease of SV30 (mL/g TSS) in the case samples was 145.25 ± 4.95 cm3/g and in the control samples, it was 70 cm3/g in the optimal condition (25).
In our study, all of the coagulants with and without the use of SMFs could have an effect on SV30. This range for samples of case 1 in the lowest level was 360 (mL/L) for FeCl3 and in the upper level was 700 (mL/L) for PACl. Furthermore, these ranges in samples of case 2 and the control in the lowest level were 460 and 920 (mL/L) and, in the upper level, they were 460 and 980 (mL/L).
3.2. Effect of Concentrations of FeCl3 with and without SMFs on SVI and pH
Effect of the combination of SMFs and different concentrations of FeCl3 in the samples of case 1 and the use of different concentrations of FeCl3 without SMFs in case 2 on SVI and pH is provided in Fig. 3.
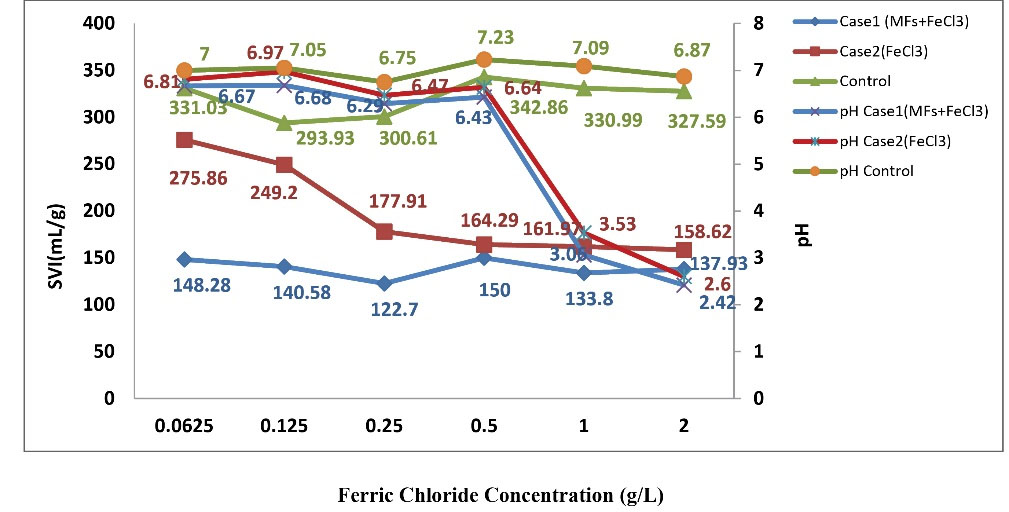
Figure 3.
Relationship between SVI, pH and, Concentration of Ferric Chloride in the Cases and Control Samples.
.
Relationship between SVI, pH and, Concentration of Ferric Chloride in the Cases and Control Samples.
Based on the result of Fig. 3, by increasing the concentration of FeCl3 in case 1 samples, no significant change was observed in the SVI (P <0.05). Therefore, the effect of SMFs on SVI is independent of the concentration of FeCl3. However, in case 2 samples, by increasing the concentration of FeCl3, the SVI was significantly decreased (P < 0.05).
When the SVI was less than 150 mL/g, the sludge bulking did not happen or could be controlled (20). In the range of 0.0625 to 2 mg/L of FeCl3 alone, this phenomenon did not happen. Nevertheless, with the use of SMFs intensity of 15 mT for 30 minutes along with FeCl3, this phenomenon occurred and sludge bulking was controlled in all samples.
FeCl3 is a salt that is commonly used for the sedimentation of flocs in AS. Fe + 3 could increase the density of flocs and this condition is suitable for sedimentation of flocs (11,12,34).
The combination of MFs and iron for sludge conditioning was studied by Hrut and Kamizela (35). The results of this study indicate the advantages of the application of MF intensity of 40 mT in dewatering sludge.
In this study, the mean decrease of SVI in case 1 (SMFs with FeCl3) was 56.71% and in case 2, it was 37.90%. Therefore, the use of SMFs along with FeCl3 and the application of FeCl3 alone could decrease SVI. It must be mentioned that the effect of MFs on SVI could be separated from the consumption of coagulants (23).
The range of Hydrogen-ion concentration for secondary treatment of wastewater is 6 to 9 and for discharge of the treated wastewater into the environment is 6.8 to 8.5 (17).
Hence, when the concentration of FeCl3 is higher than 0.5 g/L, pH adjustment is necessary. Adjusting the pH need the consumption of more alkaline, and this method raises the cost of treatment.
By increasing the concentration of FeCl3 from 0.0625 to 0.5 g/L, no change was observed in the pH of the solution, but in the range of 1 to 2 g/L, significant changes were observed.
3.3. Effect of Fe2 (SO4 )3 with and without SMFs on SVI and pH
According to Fig. 4. by consumption of Fe2(SO4)3 as a coagulant on MLSS of the effluent of aeration basins, the amount of SVI in samples of case 2 significantly lower compared to control samples, however, this change was not higher compared to when SMFs intensity of 15 mT was used. The difference in change between the samples of case 1 and case 2 samples was statistically significant. Therefore, the use of SMFs reduced the SVI from effluent of aeration basins of CMAS when mixed with Fe2(SO4)3 in comparison with case 2 and the control samples.
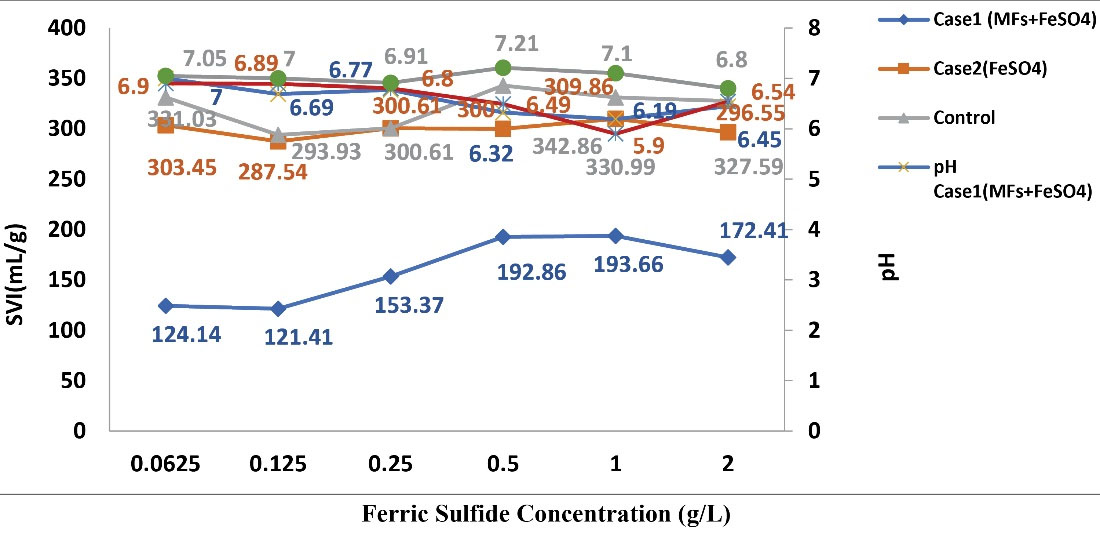
Figure 4.
Change of SVI in the Cases and Control Samples of Ferric Sulfide.
.
Change of SVI in the Cases and Control Samples of Ferric Sulfide.
By increasing the concentration of Fe2(SO4)3 from 0.0625 to 2 g/L, decrease of pH was observed. By application of Fe2(SO4)3 as coagulant, less change in pH of solution happened and, no need to adjusting the pH in the effluent of aeration basin of CMAS.
3.4. Effect of Alum With and Without SMFs on SVI and pH
Alum as a metal coagulant could reduce the SVI as illustrated in Fig. 5. However, this effect was not linear. The combination of alum and SMFs improved the reduction of SVI in case 1 when compared to case 2 and the control samples. In some samples of case 2, the amount of SVI was lower than 150 mL/g, based on the results of the independent sample t-test. By increasing the concentration of alum, no significant change in SVI was observed.
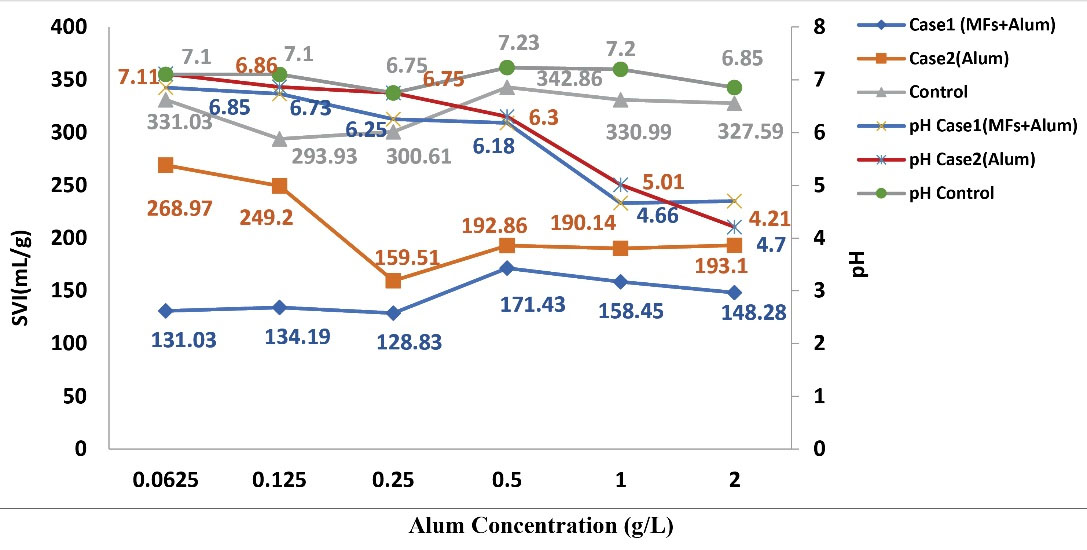
Figure 5.
Relationship between Increasing the Concentration of Alum and SVI in the Cases and Control Samples.
.
Relationship between Increasing the Concentration of Alum and SVI in the Cases and Control Samples.
Bulking control happened when the concentration of alum was lower than 0.5 (g/L) in case 1 and case 2. It is clear that only the application of alum in the range of 0.0625 to 2 (g/L) in case 2 could not control the bulking.
Increasing the concentration of alum in the range of 0.0625 to 2 g/L reduced the pH of mixed liquor in the case 1 and case 2 samples. These differences at concentrations lower than 0.25 g/L were not significant; however, at 0.5, 1, and 2 g/L concentrations, the differences were significant. In the samples of both cases at concentrations higher than 0.5 g/L, pH adjustment was necessary too. As other coagulants, alum decreased the pH of the solution. SMFs have a synergic effect on the reduction of pH when added to alum.
In 2020, Said et al (36) studied the effect of different concentrations of alum ranging from 100 to 600 mg/L on specific resistance to filtration of sludge. They mentioned that the use of 600 mg/L of alum for 60 minutes of dewatering produced better results compared to other concentrations of alum.
The effect of alum on the wastewater treatment results from the interaction between Al + 3 and extracellular polymeric substances (organic matters that are produced by microorganisms in wastewater and control the physicochemical properties of the sludge) in metal-organic complex. This phenomenon increases flocs density (34).
3.5. Effect of PACl With and Without SMFs on SVI and pH
The reduction of SVI was higher in the cases compared to the control samples, however, this phenomenon was more obvious when MFs were present. One of the interesting results of this part is that the decrease in the concentration of PACl could control bulking (less than 0.25 g/L), and at concentrations equal and higher than 0.25 g/L, control of bulking did not happen.
By increasing the concentration of PACl, a decrease in pH was observed in all samples. When the concentration of PACl was higher than 0.25 g/L, this difference was significant, and at concentrations lower than 0.25 g/L, the difference in pH change was not statistically significant between the three groups. The relationship between concentration of PACl with and without exposure of SMFs to SVI, and pH is illustrated in Fig. 6.
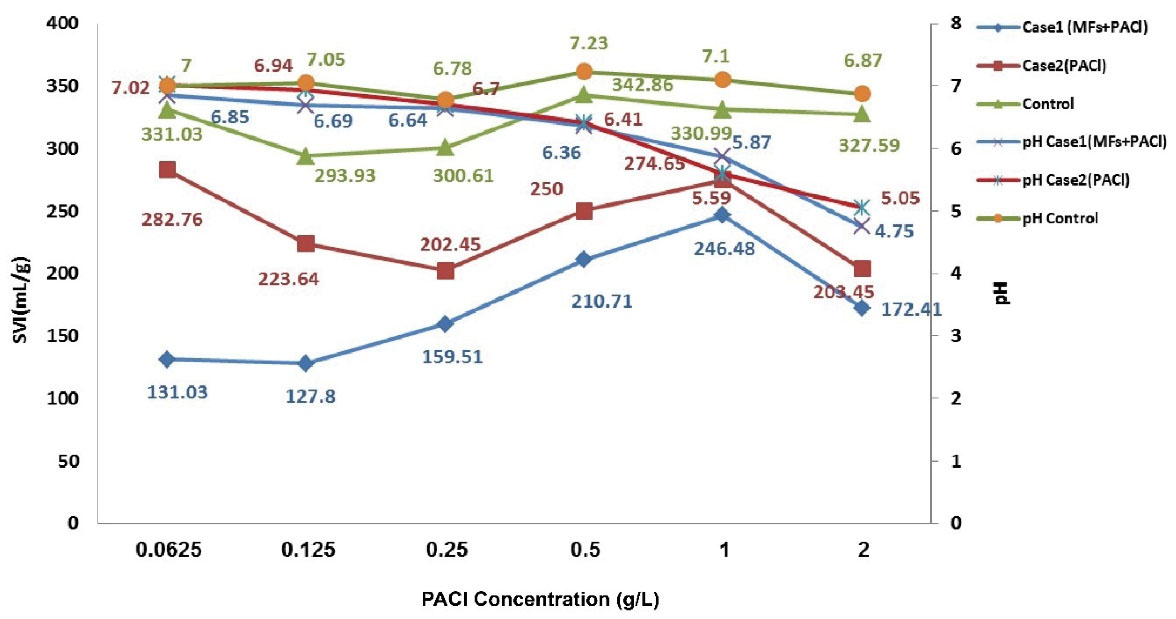
Figure 6.
Change of SVI in the Cases and Control Samples by Increasing the Concentration of PACl.
.
Change of SVI in the Cases and Control Samples by Increasing the Concentration of PACl.
The molecular weight of coagulants has a key role in bridging flocculation (37). PACl has a molecular weight of greater than 3000 Da (38). Neutralization of sludge charge and dewatering of sludge by PACl can be efficient, and this property made PACl one of the best flocculants in treatment of water and wastewater (39).
PACl has better efficiency when compared with aluminum and iron salts in the process of water and wastewater treatment, due to low temperature, low sludge volume production, and small change in pH (40,41). In our study, a small change in pH was confirmed when PACl was used as coagulants and added to effluent of aeration basin in all samples of the cases and the control.
Guo et al recommended that the optimal pH (7.5) of PACl could decrease dry solids by approximately 7.4% (42).
An important point to note is that the SMFs generate heat in the wires due to Joule heating (43). When heat was transferred to the samples, the mean temperature (ºC) rose in case 1 compared to the mean temperature in case 2 and control samples. The changes in the mean temperature of samples were summarized in Table 1.
Table 1.
Changes of Temperature in the Cases and the Control Samples
|
Coagulants
|
Mean Temperature (°C)
|
P
Value Between Case 1 and Case 2 Samples
|
P
Value Between Case 1 and Control Samples
|
P
Value Between Case 2 and Control Samples
|
|
Case 1
|
Case 2
|
Control
|
| FeCl3 |
24.35 ± 3.23 |
19.63 ± 0.94 |
19.85 ± 0.72 |
0.006* |
0.008* |
0.665 |
| Fe2(SO4)3 |
22.20 ± 2.23 |
19.50 ± 1.03 |
19.86 ± 0.85 |
0.023* |
0.038* |
0.518 |
| Alum |
24.56 ± 3.53 |
20.30 ± 1.10 |
19.83 ± 0.821 |
0.018* |
0.010* |
0.426 |
| PACL |
24.16 ± 2.97 |
20.05 ± 0.72 |
19.85 ± 0.75 |
0.008* |
0.006* |
0.648 |
* Significance level: P ≤ 0.05.
Based on Table 1, the change of temperature in case 1 compared with case 2 and the control samples was significant. However, there was no statistically significant difference in the temperature between case 2 and control samples. In this study, by 30-minute exposure of 15 mT SMFs, mean temperature of case 1 in all samples raised 3.94 °C and 3.92 °C more than those of all samples in case 2 and the control samples.
In a study, a significant decrease in SVI from 77.32 cm3/g to 20.93 cm3/g was reported at MF intensity of 48 mT in 2012 (44).
Hrut and Kamizela (35) reported that the consumption of chitosan in winter (0.5 mg/L) compared to its consumption in summer (1 mg/L) for the treatment of water in the coagulation/flocculation process is more than twice. The reason for this reaction, was related to the inverse relationship between viscosity and temperature. Another parameter is the solubility of materials in a liquid. The lower temperature is more beneficial than the higher temperature in coagulation and flocculation process (37).
3.6. Cost-Benefit of the Application of SMFs in MLSS
In this study, the SMFs exposure time of MLSS in case 1 was 30 minutes. This time is the recommended time for SV30 test. The total cost of applying the SMFs to case 1 samples and using solar panels with the same amount of energy (150 W) instead of a DC power source (alternative device for reduction of the operation cost) was estimated which is presented in Table 2.
Table 2.
Estimated Costs of the Application of SMFs in Case 1 Using DC Power and Solar Panel
|
Process
|
Unit
|
Price (US $)
|
|
DC Power (DAZHENG PS-A305D)
|
Polycrystalline Solar Panel
|
| Lacquered wire (1 mm) |
180 (m) |
25.4 |
25.4 |
| Galvanized iron sheet (0.5 mm) |
15*30 (cm) |
1 |
1 |
| Solenoid |
Rows |
10 |
10 |
| Power supply |
One device |
66 |
82.1 |
| Electricity consumption |
kWh |
0.002 |
- |
| Total cost |
US ($) |
91.502 |
107.6 |
Although the cost of polycrystalline solar panel ($16.098) is higher compared to DC power supply, it seems that the cost of using solar panel is lower than that of DC power in the long run. Furthermore, one of the main limitations of the application of solar panel is that they could not be used at absent of sunlight.
Sludge dewatering (higher density) is one of the most expensive treatment processes in WWTPs. Additionally, 80% of the operating costs are allocated to the sludge dewatering (45).Therefore, any compounding process (such as the application of SMFs) that increases the density of the sludge can be considered as a helpful method.
4. Conclusion
The main results of this study indicate the best coagulants among the four commonly used metallic chemical materials to control SVI in the effluent of aeration basin in CMAS with and without using of SMFs (Table 3).
Table 3.
Sludge Bulking Control Using a Combination of Coagulants and MFs
|
Coagulants
|
Concentration (g/L)
|
MLSS (mg/L)
|
Mean SV
30
(mL/L TSS)
|
Bulking
|
|
Control
|
Case 1
|
Case 2
|
Control
|
Case 1
|
Case 2
|
| FeCl3 |
0.0625 |
2900 |
960 |
430 |
800 |
h* |
c** |
h |
| Fe2(SO4)3 |
360 |
880 |
h |
c |
h |
| Alum |
380 |
780 |
h |
c |
h |
| PACl |
380 |
820 |
h |
c |
h |
| FeCl3 |
0.125 |
3130 |
920 |
440 |
780 |
h |
c |
h |
| Fe2(SO4)3 |
380 |
900 |
h |
c |
h |
| Alum |
420 |
780 |
h |
c |
h |
| PACl |
400 |
700 |
h |
c |
h |
| FeCl3 |
0.5 |
3260 |
960 |
420 |
460 |
h |
c |
h |
| Fe2(SO4)3 |
540 |
840 |
h |
h |
h |
| Alum |
480 |
540 |
h |
h |
h |
| PACl |
590 |
700 |
h |
h |
h |
| FeCl3 |
1 |
2800 |
940 |
380 |
460 |
h |
c |
h |
| Fe2(SO4)3 |
550 |
880 |
h |
h |
h |
| Alum |
450 |
540 |
h |
h |
h |
| PACl |
700 |
780 |
h |
h |
h |
| FeCl3 |
2 |
2840 |
950 |
400 |
460 |
h |
c |
h |
| Fe2(SO4)3 |
500 |
860 |
h |
h |
h |
| Alum |
430 |
560 |
h |
h |
h |
| PACl |
500 |
590 |
h |
h |
h |
*Happened (SVI > 150 mL/g).
**Controlled (SVI ≤ 150 mL/g).
When concentrations of coagulants were between 0.0625 to 2 g/L, and, the intensity of SMFs was 15 mT, the following results in terms of SVI were obtained:
-
Ferric chloride is the only coagulant that can control the bulking at all concentrations and, at 0.0625 and 0.125 g/L concentrations of other coagulants combined with SMFs, SVI could be lower than 150 mL/g (control of bulking happened).
-
Bulking control did not happen without exposure to SMFs.
-
All of the coagulants with and without SMFs could decrease the SVI.
-
The differences in the reduction of SVI were statistically significant in all samples of case 1 when compared to the samples of case 2.
-
When the concentrations of coagulants are equal or more than 0.5 g/L, pH adjustment is necessary for all samples.
-
With the application of SMFs, the highest reduction of SVI belonged to ferric sulfide (43.60%), followed by PACl (20.19%), alum (19.80%), and ferric chloride (18.40%).
-
Without the application of SMFs, the highest reduction of SVI belonged to ferric chloride (38.36%), followed by alum (34.94%), PACl (25.43%), and, ferric sulfide (6.69%).
Acknowledgments
The present study was financially supported by Hamadan University of Medical Sciences (Grant number: 140008186772). The experiments were done in the Wastewater Microbiology Laboratory of Sanandaj WWTP in the summer and autumn of 2021. We appreciate everyone who helped us in this program.
Conflict of Interest Disclosures
There is not any conflict of interest among the authors of this article.
Ethical Statement
This study was approved by Hamadan University of Medical Sciences (IR.UMSHA.REC.1400.586). We confirm that all procedures were conducted in accordance with the ethical standards of the Declaration of Helsinki.
References
- Liu Z, Liang Z, Wu S, Liu F. Treatment of municipal wastewater by a magnetic activated sludge device. Desalin Water Treat 2015; 53(4):909-18. doi: 10.1080/19443994.2013.848416 [Crossref] [ Google Scholar]
- Amanatidou E, Samiotis G, Bellos D, Pekridis G, Trikoilidou E. Net biomass production under complete solids retention in high organic load activated sludge process. Bioresour Technol 2015; 182:193-9. doi: 10.1016/j.biortech.2015.01.119 [Crossref] [ Google Scholar]
- Tandoi V, Rossetti S, Wanner J. Activated Sludge Separation Problems: Theory, Control Measures, Practical Experiences. IWA Publishing; 2017.
- Jorand F, Zartarian F, Thomas F, Block JC, Bottero JY, Villemin G. Chemical and structural (2D) linkage between bacteria within activated sludge flocs. Water Res 1995; 29(7):1639-47. doi: 10.1016/0043-1354(94)00350-g [Crossref] [ Google Scholar]
- Liu Y, Wang L, Ma J, Zhao X, Huang Z, Mahadevan GD. Improvement of settleability and dewaterability of sludge by newly prepared alkaline ferrate solution. Chem Eng J 2016; 287:11-8. doi: 10.1016/j.cej.2015.11.037 [Crossref] [ Google Scholar]
- Qi Y, Thapa KB, Hoadley AFA. Application of filtration aids for improving sludge dewatering properties–a review. Chem Eng J 2011; 171(2):373-84. doi: 10.1016/j.cej.2011.04.060 [Crossref] [ Google Scholar]
- Zhai LF, Sun M, Song W, Wang G. An integrated approach to optimize the conditioning chemicals for enhanced sludge conditioning in a pilot-scale sludge dewatering process. Bioresour Technol 2012; 121:161-8. doi: 10.1016/j.biortech.2012.06.093 [Crossref] [ Google Scholar]
- Feng HJ, Hu LF, Qiu CD, Shen DS. [Influence on three categories of metal ions on adsorption of humic acid by activated sludge]. Huan Jing Ke Xue 2008; 29(1):77-81. [ Google Scholar]
- Matsui Y, Matsushita T, Sakuma S, Gojo T, Mamiya T, Suzuoki H. Virus inactivation in aluminum and polyaluminum coagulation. Environ Sci Technol 2003; 37(22):5175-80. doi: 10.1021/es0343003 [Crossref] [ Google Scholar]
- Zhang L, Zheng J, Tian S, Zhang H, Guan X, Zhu S. Effects of Al3 + on the microstructure and bioflocculation of anoxic sludge. J Environ Sci (China) 2020; 91:212-21. doi: 10.1016/j.jes.2020.02.010 [Crossref] [ Google Scholar]
- Tabaki S, Ardestani F. Evaluation of Natural and Chemical Coagulants Performance in Treatment of Municipal Wastewater of Behshahr City. Avicenna Journal of Environmental Health Engineering 2021; 8(2):110-5. doi: 10.34172/ajehe.2021.14 [Crossref] [ Google Scholar]
- Shrestha S, Sharma K, Chen Z, Yuan Z. Unravelling the influences of sewer-dosed iron salts on activated sludge properties with implications on settleability, dewaterability and sludge rheology. Water Res 2019; 167:115089. doi: 10.1016/j.watres.2019.115089 [Crossref] [ Google Scholar]
- Chen Z, Zhang W, Wang D, Ma T, Bai R. Enhancement of activated sludge dewatering performance by combined composite enzymatic lysis and chemical re-flocculation with inorganic coagulants: kinetics of enzymatic reaction and re-flocculation morphology. Water Res 2015; 83:367-76. doi: 10.1016/j.watres.2015.06.026 [Crossref] [ Google Scholar]
- Wang JP, Chen YZ, Wang Y, Yuan SJ, Yu HQ. Optimization of the coagulation-flocculation process for pulp mill wastewater treatment using a combination of uniform design and response surface methodology. Water Res 2011; 45(17):5633-40. doi: 10.1016/j.watres.2011.08.023 [Crossref] [ Google Scholar]
- Zouboulis AI, Moussas PA, Tzoupanos ND. Coagulation-flocculation processes applied in water or wastewater treatment. In: Samuelson JP, ed. Industrial Waste: Environmental Impact, Disposal and Treatment. Hauppauge: Nova Science Publishers; 2009. p. 289-324.
- Oller I, Malato S, Sánchez-Pérez JA. Combination of advanced oxidation processes and biological treatments for wastewater decontamination--a review. Sci Total Environ 2011; 409(20):4141-66. doi: 10.1016/j.scitotenv.2010.08.061 [Crossref] [ Google Scholar]
- Almeida F, Grzebielucka EC, Antunes SRM, Borges CPF, Andrade AVC, Souza É CF. Visible light activated magnetic photocatalysts for water treatment. J Environ Manage 2020; 273:111143. doi: 10.1016/j.jenvman.2020.111143 [Crossref] [ Google Scholar]
- Abou Hammad AB, Hemdan BA, El Nahrawy AM. Facile synthesis and potential application of Ni0.6Zn0.4Fe.2O4 and Ni0.6Zn0.2Ce0.2Fe.2O4 magnetic nanocubes as a new strategy in sewage treatment. J Environ Manage 2020; 270:110816. doi: 10.1016/j.jenvman.2020.110816 [Crossref] [ Google Scholar]
- Zaidi NS, Sohaili J, Muda K, Sillanpää M. Magnetic field application and its potential in water and wastewater treatment systems. Sep Purif Rev 2014; 43(3):206-40. doi: 10.1080/15422119.2013.794148 [Crossref] [ Google Scholar]
- Tchobanoglous G, Stensel HD, Tsuchihashi R, Burton FL, Abu-Orf M, Bowden G, et al. Wastewater Engineering: Treatment and Resource Recovery. New York, NY: McGraw-Hill Education; 2014.
- Parker EN. Cosmical Magnetic Fields: Their Origin and Their Activity. Oxford: Oxford University Press; 2019.
- Zhao B, Sha H, Li J, Cao S, Wang G, Yang Y. Static magnetic field enhanced methane production via stimulating the growth and composition of microbial community. J Clean Prod 2020; 271:122664. doi: 10.1016/j.jclepro.2020.122664 [Crossref] [ Google Scholar]
- Asgari G, Khoshniyat R, Nazifi M, Shabrandi H. Investigation of the effect of magnetic fields on the volume index of diluted municipal wastewater sludge. J Water Wastewater 2021; 32(3):113-24. doi: 10.22093/wwj.2020.254929.3076.[Persian] [Crossref] [ Google Scholar]
- Mateescu C, Buruntea N, Stancu N. Investigation of Aspergillus niger growth and activity in a static magnetic flux density field. Rom Biotechnol Lett 2011; 16(4):6364-8. [ Google Scholar]
- Zieliński M, Rusanowska P, Dębowski M, Hajduk A. Influence of static magnetic field on sludge properties. Sci Total Environ 2018; 625:738-42. doi: 10.1016/j.scitotenv.2017.12.226 [Crossref] [ Google Scholar]
- Edzwald JK, Haarhoff J. Seawater pretreatment for reverse osmosis: chemistry, contaminants, and coagulation. Water Res 2011; 45(17):5428-40. doi: 10.1016/j.watres.2011.08.014 [Crossref] [ Google Scholar]
- Yu W, Gregory J, Campos LC. Breakage and re-growth of flocs formed by charge neutralization using alum and polyDADMAC. Water Res 2010; 44(13):3959-65. doi: 10.1016/j.watres.2010.04.032 [Crossref] [ Google Scholar]
- Letterman RD. Water Quality and Treatment: A Handbook of Community Water Supplies. New York: McGraw-Hill; 1999.
- Altaher H, ElQada E, Omar W. Pretreatment of wastewater streams from petroleum/petrochemical industries using coagulation. Adv Chem Eng Sci 2011; 1(4):245-51. doi: 10.4236/aces.2011.14035 [Crossref] [ Google Scholar]
- Niu M, Zhang W, Wang D, Chen Y, Chen R. Correlation of physicochemical properties and sludge dewaterability under chemical conditioning using inorganic coagulants. Bioresour Technol 2013; 144:337-43. doi: 10.1016/j.biortech.2013.06.126 [Crossref] [ Google Scholar]
- Wang HF, Hu H, Wang HJ, Zeng RJ. Impact of dosing order of the coagulant and flocculant on sludge dewatering performance during the conditioning process. Sci Total Environ 2018; 643:1065-73. doi: 10.1016/j.scitotenv.2018.06.161 [Crossref] [ Google Scholar]
- Lin Q, Peng H, Zhong S, Xiang J. Synthesis, characterization, and secondary sludge dewatering performance of a novel combined silicon-aluminum-iron-starch flocculant. J Hazard Mater 2015; 285:199-206. doi: 10.1016/j.jhazmat.2014.12.005 [Crossref] [ Google Scholar]
- Yang R, Li H, Huang M, Yang H, Li A. A review on chitosan-based flocculants and their applications in water treatment. Water Res 2016; 95:59-89. doi: 10.1016/j.watres.2016.02.068 [Crossref] [ Google Scholar]
- Yang P, Li D, Zhang W, Wang N, Yang Z, Wang D. Flocculation-dewatering behavior of waste activated sludge particles under chemical conditioning with inorganic polymer flocculant: effects of typical sludge properties. Chemosphere 2019; 218:930-40. doi: 10.1016/j.chemosphere.2018.11.169 [Crossref] [ Google Scholar]
- Hrut K, Kamizela T. Changes in filtration properties of digested sludge under the influence of magnetic field. Desalin Water Treat 2018; 117:282-9. [ Google Scholar]
- Said MA, Shihab MS, Hamid AT. Dewatering of wastewater sludge using alum Fresenius Environ. Bull 2020; 29(12):10659-67. [ Google Scholar]
- Zheng H, Sun Y, Guo J, Li F, Fan W, Liao Y. Characterization and evaluation of dewatering properties of PADB, a highly efficient cationic flocculant. Ind Eng Chem Res 2014; 53(7):2572-82. doi: 10.1021/ie403635y [Crossref] [ Google Scholar]
- Gao BY, Chu YB, Yue QY, Wang BJ, Wang SG. Characterization and coagulation of a polyaluminum chloride (PAC) coagulant with high Al13 content. J Environ Manage 2005; 76(2):143-7. doi: 10.1016/j.jenvman.2004.12.006 [Crossref] [ Google Scholar]
- Pambou YB, Fraikin L, Salmon T, Crine M, Léonard A. Enhanced sludge dewatering and drying comparison of two linear polyelectrolytes co-conditioning with polyaluminum chloride. Desalin Water Treat 2016; 57(58):27989-8006. doi: 10.1080/19443994.2016.1178602 [Crossref] [ Google Scholar]
- Yu J, Wang D, Yan M, Ye C, Yang M, Ge X. Optimized coagulation of high alkalinity, low temperature and particle water: pH adjustment and polyelectrolytes as coagulant aids. Environ Monit Assess 2007; 131(1-3):377-86. doi: 10.1007/s10661-006-9483-3 [Crossref] [ Google Scholar]
- Yang Z, Gao B, Cao B, Xu W, Yue Q. Effect of OH−/Al3 + ratio on the coagulation behavior and residual aluminum speciation of polyaluminum chloride (PAC) in surface water treatment. Sep Purif Technol 2011; 80(1):59-66. doi: 10.1016/j.seppur.2011.04.007 [Crossref] [ Google Scholar]
- Guo J, Nengzi L, Zhao J, Zhang Y. Enhanced dewatering of sludge with the composite of bioflocculant MBFGA1 and P(AM-DMC) as a conditioner. Appl Microbiol Biotechnol 2015; 99(7):2989-98. doi: 10.1007/s00253-015-6401-z [Crossref] [ Google Scholar]
- Agarwal KB, Nassif SR, Rose RD, Xu C. Rapid Estimation of Temperature Rise in Wires Due to Joule Heating. Google Patents; 2014.
- Wang XH, Diao MH, Yang Y, Shi YJ, Gao MM, Wang SG. Enhanced aerobic nitrifying granulation by static magnetic field. Bioresour Technol 2012; 110:105-10. doi: 10.1016/j.biortech.2012.01.108 [Crossref] [ Google Scholar]
- Wang Z, Wu Z, Hua J, Wang X, Du X, Hua H. Application of flat-sheet membrane to thickening and digestion of waste activated sludge (WAS). J Hazard Mater 2008; 154(1-3):535-42. doi: 10.1016/j.jhazmat.2007.10.057 [Crossref] [ Google Scholar]