Avicenna J Environ Health Eng. 8(1):52-60.
doi: 10.34172/ajehe.2021.08
Original Article
Equilibrium Isotherms and Kinetics Modelling for an Efficient Removal of 4-Chloro-2-Methoxyphenol From Aqueous Solution Using Optimal Activated Carbon
Zaharaddeen N. Garba 1, *  , I. Ibrahim 1, Afidah Abdul Rahim 2
, I. Ibrahim 1, Afidah Abdul Rahim 2
Author information:
1Department of Chemistry, Ahmadu Bello University, Zaria, Nigeria
2School of Chemical Sciences, Universiti Sains Malaysia, Penang, Malaysia
Abstract
A surface area of 1085.92 m2 /g and a monolayer adsorption capacity of 497.66 mg/g were obtained from the optimum activated carbon derived using Prosopis africana seed hulls (PASH-AC) at the activation temperature of 795°C, activation time of 62 minutes, and impregnation ratio of 2.45. Five different forms of the linearized Langmuir equations along with two other models (Freundlich and Temkin) were tested on the adsorption data. The best adsorption model was selected using correlation coefficient (R2 ) and chi-square (χ2 ) was used for assessing the validity of each isotherm model. Langmuir-2 along and pseudo-second-order models were found to be the most suitable model for describing the equilibrium and kinetic processes, respectively.
Keywords: Prosopis africana seed hulls, Activated carbon, Isotherms and kinetics modelling, Adsorption, 4-Chloro-2-methoxy phenol
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
In recent times, the pollution of water is among the main challenges of the environment distressing the modern world (1-7). Organic-based chemical contaminants such as chlorophenols are among water pollutants. Pharmacological similarities exist between chemical contaminants such as guaiacols and phenol. Chlorinated forms of guaiacols are quite similar to chlorophenols. They pose severe health hazards by affecting human nervous as well as respiratory systems. They have a strong odor, are not readily biodegradable, and are persistent in the environment. Additionally, they are toxic and have carcinogenic features (8,9)
One of the most valuable and effective control technologies for wastewater treatment is adsorption (10-17). Activated carbon (AC) is the best practical adsorbent due to its easy operation as well as good adsorption capacity (18-21). ACs are considered adsorbents as a result of their adaptable exterior properties which are broadly applied for a wide range of industries (12,19,22-24). The transformation of unwanted material from agriculture into valuable products towards the elimination of a possible pollutant in a cost-effective and eco-friendly manner has been suggested as an ingenious technique. Our previous studies have described the optimal conditions for AC preparations using Prosopis africana seed hulls (PASH) (25-27) but no report on the removal of 4-chloro-2-methoxy phenol (4C2MP) from synthetic wastewater using CH3COONa impregnated PASH-AC was found in the literature.
This work is therefore aimed at investigating the influence of the initial concentration of 4C2MP, time of adsorption, and solution pH on the PASH-AC with sodium acetate (CH3COONa) utilized as the activating chemical agent to remove 4C2MP. Other investigations carried out on the PASH-AC are based on equilibrium data modelling, kinetics, and thermodynamics study.
2. Materials and Methods
2.1. Preparation and Characterization of Adsorbent (PASH-AC)
The precursor (PASH) used in this study was prepared in Zaria, Nigeria, using a similar optimization technique applied in our earlier work (25). The AC was prepared using sodium acetate as an activating agent, and the dried precursor (of the desired particle size) was measured and mixed with the activating agent at different ratios. The mixture was heated in a water bath (80–90°C) overnight and dried in an oven for 24 hours to remove moisture before loading into a furnace embedded in a tubular reactor. The carbonization step involved heating the reactor at 700°C under purified nitrogen (99.99%) atmosphere with the flow rate of 150 mL/min. The carbonized material was then activated under CO2 gas at a flow rate of 150 mL/min using a reactor similar to the one used for carbonization under different temperatures and held for varying periods of time. The product was cooled to room temperature, washed with distilled water until a neutral pH was attained, oven dried, and finally stored in an airtight container for further use. Similar optimal preparation conditions (activation temperature of 795°C, activation time of 62 minutes, and impregnation ratio of 2.45) as reported in our earlier work were applied (25) which produced PASH-AC with reasonable yield, thereby removing a significant amount of 4C2MP.
Scanning electron microscopy (SEM) analysis was carried out on the AC prepared at the optimum conditions using FEI QUANTA PEG 60 model to study its surface morphology with the surface functional groups of the adsorbent detected using FTIR spectroscopy (FTIR-2000, Perkin Elmer, GX infrared spectroscopy) and spectra were recorded at 4000-400 cm-1 range.
The pH point of zero charge (pHpzc) was determined by adopting solid addition method similar to the method which involved the addition of adsorbent sample (0.1 g) into 100 mL conical flasks containing
50 mL of various concentrations of KNO3 (0.1, 0.01, 0.001 mol dm−3). To adjust the initial pH of the KNO3 solution from 2 to 12, 0.1 M KOH and HCl were used. The sample was shaken at 180 rpm until equilibrium was established, and the final pH was recorded. The final pH was plotted against initial pH values of the solution with plateau point on the plot noted and recorded as the pHpzc of the adsorbent.
2.2. Batch Adsorption of 4C2MP
Experiments were carried out using batch adsorption to remove 4C2MP by PASH-AC as reported previously (25).
At equilibrium (% R), the percentage of 4C2MP removed was estimated as:
(1)
where Co and Ce (mg/L) represent the initial and equilibrium concentrations, respectively.
The equilibrium amount of 4C2MP adsorbed, qe (mg/g), was evaluated by equation 2:
The kinetics of adsorption process was analysed by estimating 4C2MP concentration at different times. The degree of 4C2MP adsorbed at time t, qt (mg/g), was estimated by equation 3:
Solutions pH as well as functional group were determined using the same process as reported in our earlier published work (11).
3. Results and Discussion
3.1. Characterization of PASH-AC
Figs. 1a and 1b show the SEM images of the raw PASH and the PASH-AC, respectively.
The surface texture of the precursor was rough, uneven, undulating, and porous. As shown in Fig. 1b, homogeneous porous structures were distributed on the surface of the PASH-AC. This result revealed that the combined activation process of CH3COONa and CO2 was effective in creating well-developed pores, resulting in AC with a large surface area and a good mesoporous structure. Other researchers have described similar observations on coconut husk (28), Borassus aethiopum shells (29), oil palm shell (30), and Canarium schweinfurthii seed shell (12).
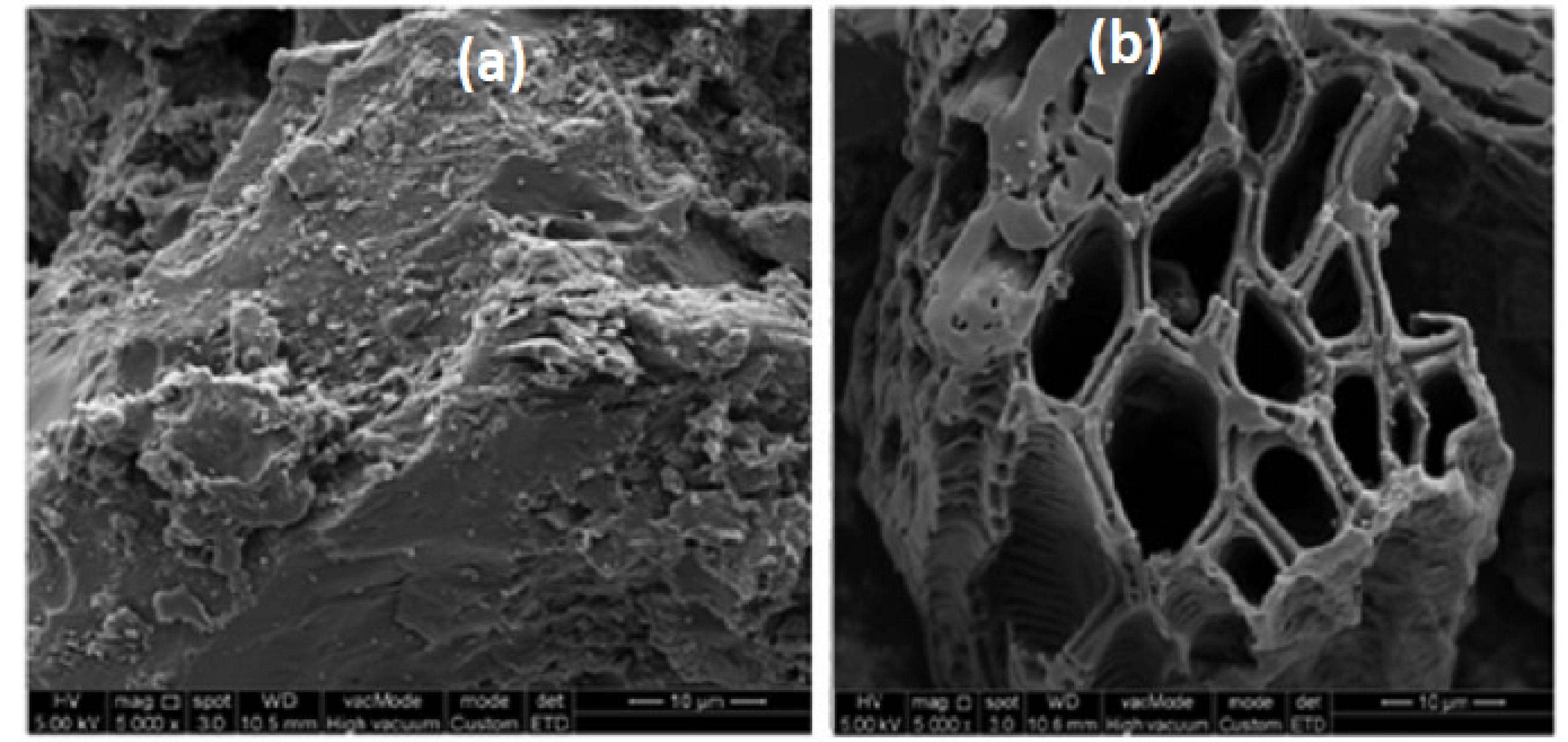
Figure 1.
SEM Micrograph of (a) Raw PASH Sample and (b) PASH-Based Activated Carbon.
.
SEM Micrograph of (a) Raw PASH Sample and (b) PASH-Based Activated Carbon.
A disparity can be observed upon comparing the PASH and PASH-AC spectra (Fig. 2).
The broad bands observed at 3500-3200 cm-1, 3300-3000 cm-1 and 3000-2800 cm-1 correspond to O-H, N-H and both saturated as well as unsaturated C-H groups, respectively. The feeble peak observed around 700-800 cm-1was assigned to C-OH (out of plane bending) in phenol. Carbonization followed by activation was responsible for the disappearance of several functional groups as observed in the PASH-AC spectra. This observation was ascribed to the effect of thermal degradation, which led to the obliteration of intermolecular bonding.
The pHpzc of the adsorbent was found to be 6.53, revealing that the surface area of the adsorbent was larger on the acidic group. A similar observation was reported by other researchers (31).
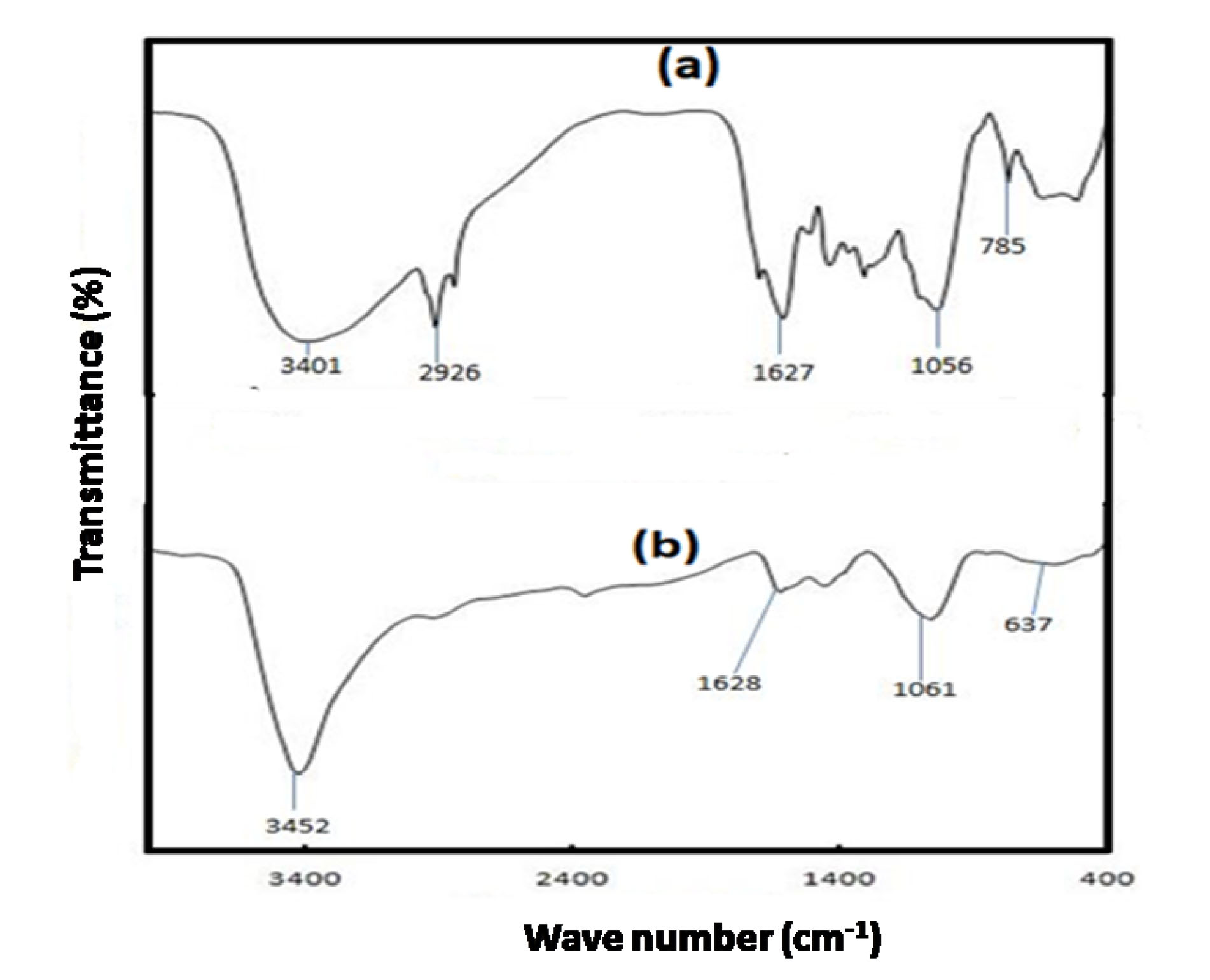
Figure 2.
FT-IR Spectra of (a) PASH and (b) PASH-AC.
.
FT-IR Spectra of (a) PASH and (b) PASH-AC.
3.2. Impact of the Concentration of 4C2MP and the Time of Adsorption
A fast increase in 4C2MP concentrations can be observed in Fig. 3a, followed by a slow uptake until equilibrium, which was attained at minimum time for lower initial concentrations. The variance in equilibrium time realization was ascribed to the quicker elimination or vanishing of adsorbate molecules at dissimilar adsorbate concentrations (31)
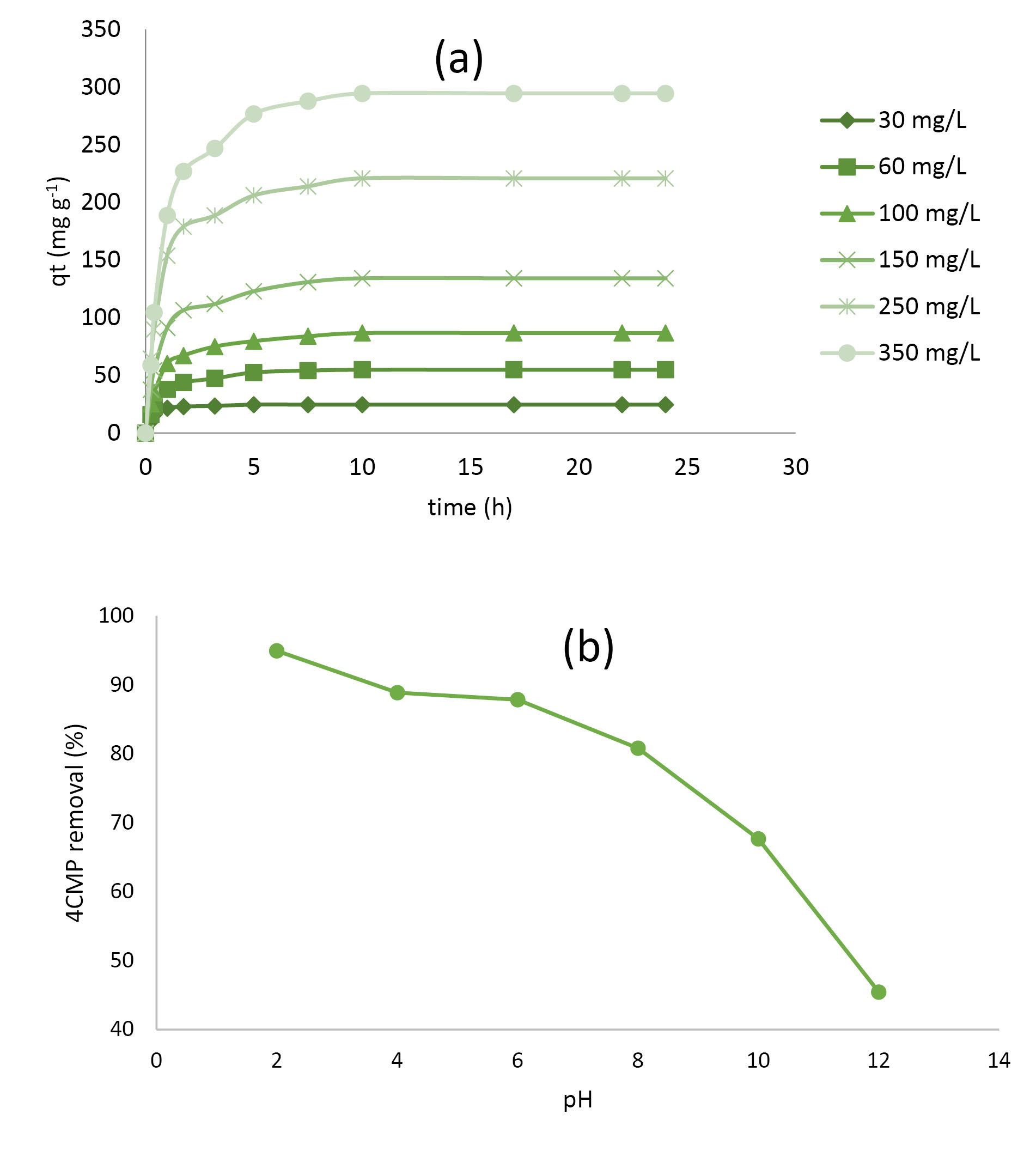
Figure 3.
(a)Effect of Contact Time on 4C2MP Adsorption Onto PASH-AC at Various Initial Concentrations (b) Effect of Solution pH on 4C2MP Removal by PASH-AC.
.
(a)Effect of Contact Time on 4C2MP Adsorption Onto PASH-AC at Various Initial Concentrations (b) Effect of Solution pH on 4C2MP Removal by PASH-AC.
3.3 Influence of Solution pH
As depicted in Fig. 3b, the percentage of 4C2MP removal indicated a substantial decline with a rise in the pH of the solution from 2 to 12. At pH 2. A removal percentage of 95.81% was attained, which was attributed to its great affinity to form hydrogen bonding with the external surface of the PASH-AC as a result of the methoxy group asserting the influence of withdrawing group (32).
3.4. Adsorption Isotherms Modelling
Langmuir (L1-L5), Freundlich (F), and Temkin (T) isotherm models were used to investigate the equilibrium data.
Langmuir isotherm is widely used for the elimination of water pollutants, described by equation 4 (33):
The constants of the isotherm linked to the adsorption capacity and rate were represented as (mg/g) and KL (L/mg), respectively. Equation 4 was conveyed in five dissimilar linear forms, as presented in Table 1, with great discrepancies associated with the dispersal of the data and the accuracy of parameter determination (34).
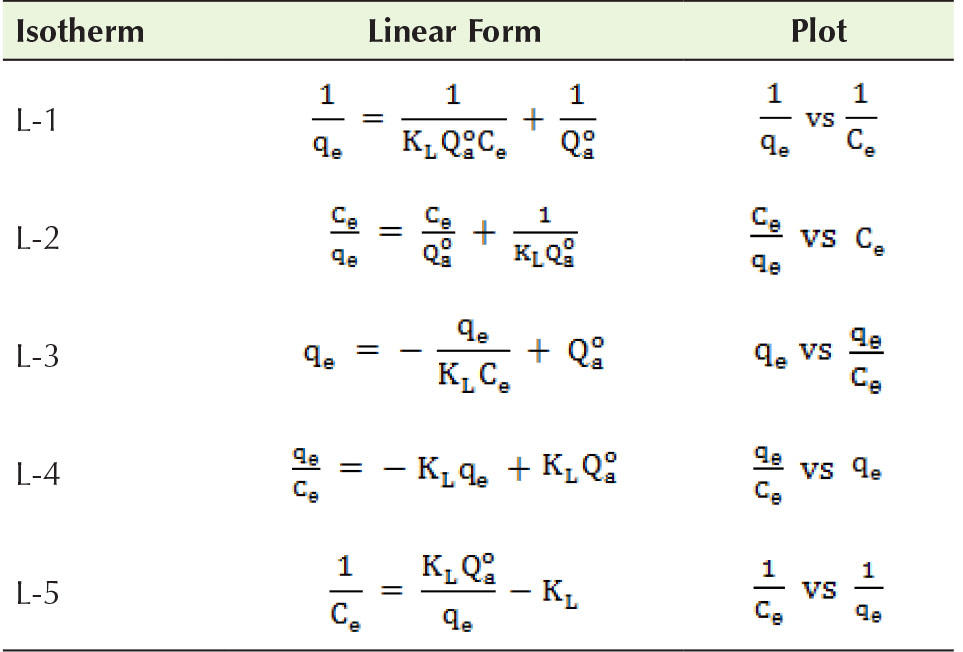
Table 1 Linear Forms of Langmuir Isotherm.
.
Another important adsorption parameter called dimensionless separation factor (RL) is defined as (35):
where Co denotes the concentration of 4C2MP. RL values indicate whether the adsorption is unfavourable (RL > 1), linear (RL = 1), favourable (0 < RL < 1), or irreversible (RL = 0).
The adsorption process on a surface that is heterogeneous is described by Freundlich (F) isotherm which is described as (36):
where KF and n stand for adsorption capacity deviation of the model from linearity, respectively. Generally, the higher the n value, the more favourable the adsorption process.
The third model applied to the generated adsorption data was Temkin (T) model, depicted as (37):
For the proper understanding of the model legitimacy in explaining the process, χ2 was calculated and considered due to uncertainty about the use of correlation coefficient (R2) only as it is no longer reliable in selecting the most suitable model since it only explains the correlation between linear forms of the isotherm equations and experimental data with χ2best describing the appropriateness between predicted and experimental values of the adsorption capacity. Better fit is indicated by the lower χ2value.
As can be observed in Table 2, there are varying values deducted from the five linear L equations, because the original error distribution was altered by the transformations (34).
Table 2.
Langmuir (L-1 to L-5), Freundlich (F), and Temkin (T) Isotherm Model Parameters (Correlation Coefficients and Chi-square Values) for 4C2MP Adsorption on PASH-AC at 30◦C
|
Langmuir
|
Isotherm Parameters
|
|
Qa0 (mg/g)
|
RL
|
R
2
|
χ
2
|
| L-1 |
334.21 |
0.045 |
0.9920 |
4.380 |
| L-2 |
497.66 |
0.074 |
0.9665 |
0.393 |
| L-3 |
449.48 |
0.064 |
0.8625 |
2.475 |
| L-4 |
498.54 |
0.073 |
0.8625 |
9.740 |
| L-5 |
343.56 |
0.046 |
0.9920 |
5.725 |
| F |
KF (mg/g (L/mg) |
n |
R2 |
χ2 |
|
|
22.30 |
1.382 |
0.9876 |
1.420 |
| T |
A (L/g) |
B (J/mol) |
R2 |
χ2 |
|
|
0.620 |
84.385 |
0.9281 |
31.750 |
L-1 or L-5 was determined to be the most suitable isotherm to describe the adsorption process based on the R2 values obtained since they exhibited the largest values (R2 = 0.9920). However, higher R2values are no longer reliable in describing the best transformations according to (34). As can clearly be seen in Table 2, the highest R2 values (R2 = 0.9920) were exhibited by L-1 and L-5, however, the χ2values shown by those models were high, larger than 0.393 obtained from L-2. Therefore, L-1 and L-5 cannot be reliable in perfectly describing the equilibrium data. Another important observation from Table 2 is that the χ2values obtained from F (1.420) as well as T (31.750) were also greater than 0.393 obtained from the L-2 isotherm, indicating that it was the most reliable model in describing the adsorption process. Therefore, 497.67 mg/g and 0.074 were selected as the correct values for the maximum adsorption capacity (and separation factor (RL), respectively, which were picked up from the L-2 equation.
The high value (497.67 mg/g) adopted in this work was ascribed to the mesoporous nature as well as the large surface area of PASH-AC (25). It performed very well upon comparison with values compiled from previous researchers in Table 3.
Table 3.
Comparison of Maximum Monolayer Adsorption Capacity of Various Chlorophenols on Different Adsorbents
|
Adsorbent
|
Adsorbate
|
(mg/g)
|
Reference
|
| PASH-AC |
4-Chloro2-methoxy phenol |
497.66 |
This work |
| Commercial activated carbon |
4-Chloro2-methoxy phenol |
276.88 |
This study |
| Oil palm shell activated carbon |
4-Chloro2-methoxy phenol |
323.62 |
(32) |
| Oil palm shell activated carbon |
4-Chloroguaiacol |
454.45 |
(30) |
| Rattan sawdust based activated carbon |
4-chlorophenol |
188.68 |
(38) |
| Cattail fiber-based activated carbon |
2,4-Dichlorophenol |
142.86 |
(39) |
| Rice straw carbon |
3-chlorophenol |
14.2 |
(40) |
3.5. Adsorption Kinetic Studies
Lagergren pseudo-first-order and pseudo-second-order (1 and 2) were the popular kinetic models applied in studying the 4C2MP adsorption process. The linear equation of pseudo-first-order was given as (41):
(8)
The pseudo-second-order equations were depicted in two forms as in equations 9 and 10:
All the values obtained from the linear plots of equation 8 (Fig. 4a) as well as equations 9 and 10 (Fig. 4b and 4c) for the 4C2MP adsorption process were tabulated (Table 4). There was no coherence in the trends shown by the R2 values obtained from the pseudo-first-order model (0.741-0.952). In addition, there was a poor correlation between the calculated as well as the experimental qe values, indicating the unsuitability of the model for properly explaining the process of 4C2MP adsorption onto PASH-AC. However, similarity was observed when the qe values (calculated and experimental) deducted from pseudo-second-order models were compared with each other, indicating that almost all the obtained R2 values were very close to one, thereby re-affirming the suitability of pseudo-second-order model as the most reliable kinetic model in describing the adsorption process of 4C2MP onto PASH-AC. Furthermore, the lower the χ2 values (0.001-1.999) obtained from both the pseudo-second-order 1 and 2 models upon comparison with those acquired from the pseudo-first-order (11.164-59.109) further confirmed the suitability of pseudo-second-order equation as the best kinetic model to describe the 4C2MP adsorption onto PASH-AC.The failure of kinetic models in the description as well as proper detection of diffusion
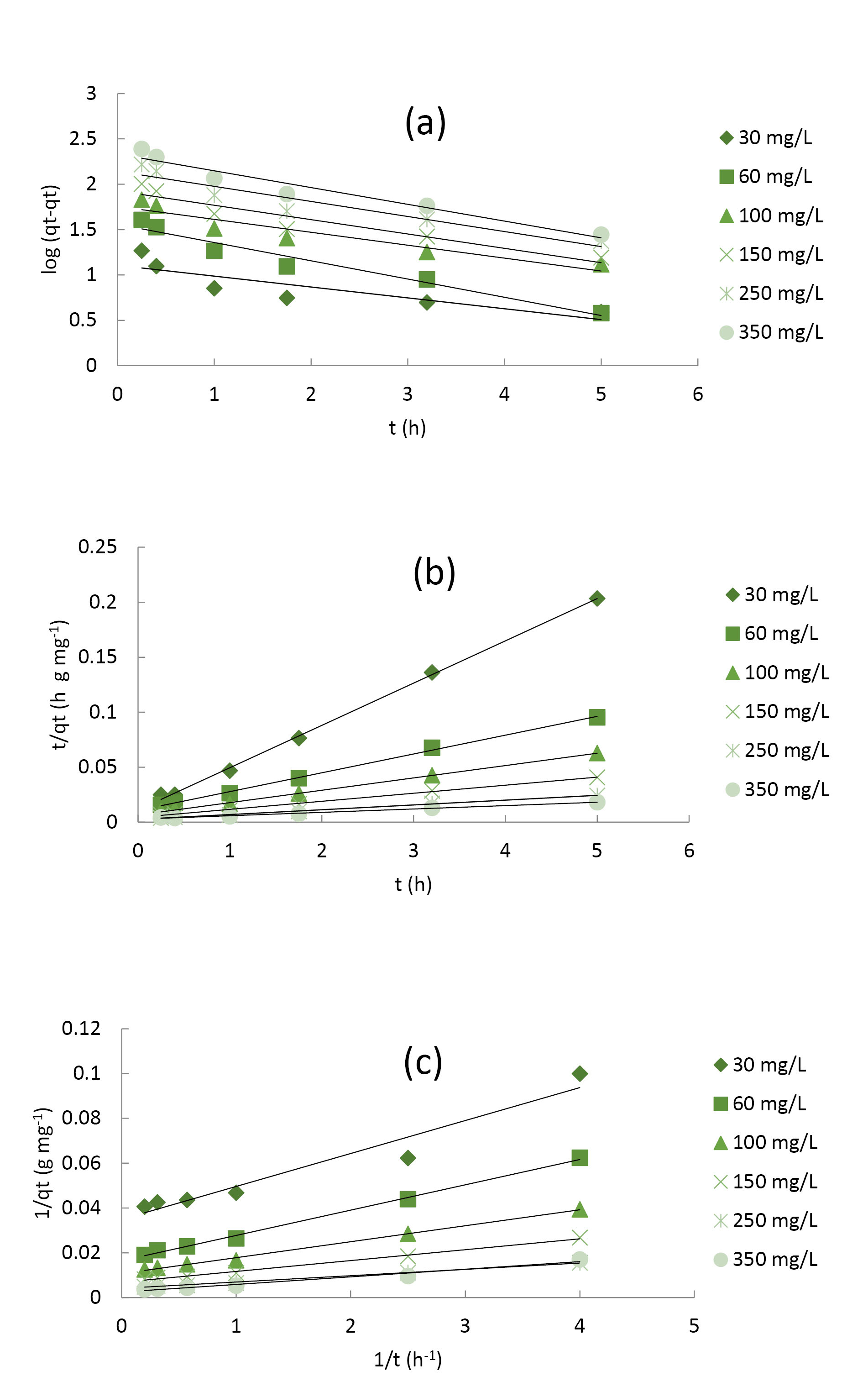
Figure 4.
Linearized Plots of (a) Pseudo-First-Order (b) Pseudo-Second-Order 1 and (c) Pseudo Second-Order 2 Kinetic Models for 4C2MP Adsorption on PASH-AC at 30°C.
.
Linearized Plots of (a) Pseudo-First-Order (b) Pseudo-Second-Order 1 and (c) Pseudo Second-Order 2 Kinetic Models for 4C2MP Adsorption on PASH-AC at 30°C.
Table 4.
Pseudo-First-Order and Pseudo-Second-Order (1 & 2) Kinetic Model Parameters of 4C2MP Adsorption on PASH-AC at 30°C
|
C
o
(mg/L)
|
q
e,exp
(mg/g)
|
Pseudo-First-Order
|
Pseudo-Second-Order-1
|
Pseudo-Second-Order-2
|
|
k
1
(1/h)
|
q
e, cal
(mg/g)
|
R
2
|
χ
2
|
k
2
(g/mg h)
|
q
e, cal
(mg/g)
|
R
2
|
χ
2
|
k
2
(g/mg h)
|
q
e, cal
(mg/g)
|
R
2
|
χ
2
|
| 30 |
28.51 |
0.276 |
12.78 |
0.741 |
19.361 |
0.134 |
26.04 |
0.999 |
0.234 |
0.083 |
28.65 |
0.943 |
0.001 |
| 60 |
56.29 |
0.463 |
36.19 |
0.952 |
11.164 |
0.028 |
58.48 |
0.998 |
0.082 |
0.024 |
60.98 |
0.997 |
0.361 |
| 100 |
92.83 |
0.328 |
56.95 |
0.889 |
22.605 |
0.019 |
89.29 |
0.999 |
0.140 |
0.016 |
93.46 |
0.996 |
0.004 |
| 150 |
138.47 |
0.366 |
84.47 |
0.895 |
34.521 |
0.012 |
136.99 |
0.998 |
0.016 |
0.094 |
147.06 |
0.994 |
0.502 |
| 250 |
229.63 |
0.383 |
138.99 |
0.898 |
59.109 |
0.008 |
227.27 |
0.998 |
0.025 |
0.006 |
243.90 |
0.996 |
0.835 |
| 350 |
304.75 |
0.426 |
215.38 |
0.942 |
37.083 |
0.003 |
322.58 |
0.993 |
0.986 |
0.002 |
330.45 |
0.974 |
1.999 |
mechanisms prompted intraparticle diffusion model to be applied, which was mathematically described as:
As shown in Fig. 5, there was very rapid adsorption at the beginning attributed to the electrostatic attraction which was strong between 4C2MP and the external surface of PASH-AC.
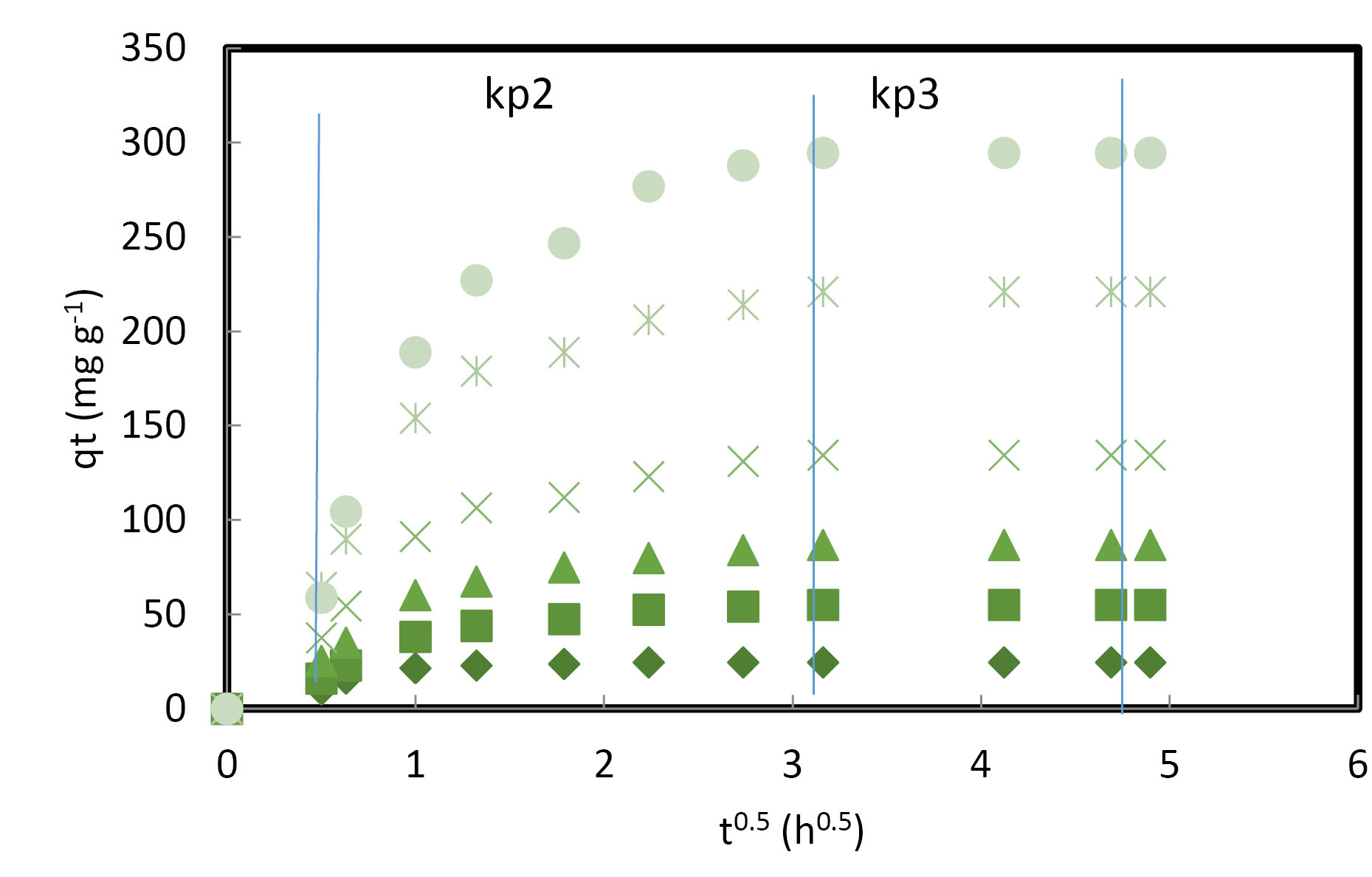
Figure 5.
Plot of Intraparticle Diffusion Model for Adsorption of 4C2MP onto PASH-AC at 30oC.
.
Plot of Intraparticle Diffusion Model for Adsorption of 4C2MP onto PASH-AC at 30oC.
That was followed by a stage of steady adsorption, ascribed to intraparticle diffusion of the 4C2MP molecule through the adsorbent. In some cases, when the intraparticle diffusion begins to slow down, a third stage exists. The start stage is usually possible when the initial concentration of the adsorbate is high (42).
An increase in the kp2 values can be noticed in Table 5 which keeps rising with an increase in the initial concentration of 4C2MP. That was attributed to a strong driving force. Furthermore, there was an upsurge in the values of C2 and C3 with the increase in 4C2MP concentration from 30 to 350 mg/L, suggesting the boundary layer to be thicker (43). It can also be observed from Fig. 5 with respect to the second and third stages that the linear lines evaded the origin.
Table 5.
Intraparticle Diffusion Model Parameters for the Adsorption of 4C2MP onto PASH-AC
|
C
o
(mg/L)
|
Intraparticle Diffusion Model
|
|
k
p2
(mg/g h
1/2
)
|
k
p3
(mg/g h
1/2
)
|
C
2
|
C
3
|
(R
2
)
2
|
(R
3
)
2
|
| 30 |
7.142 |
- |
10.852 |
24.610 |
0.7280 |
- |
| 60 |
20.072 |
0.2227 |
11.723 |
53.843 |
0.8828 |
0.4919 |
| 100 |
30.457 |
0.9199 |
19.147 |
82.518 |
0.8662 |
0.4919 |
| 150 |
46.831 |
1.1000 |
29.012 |
129.240 |
0.8563 |
- |
| 250 |
78.519 |
2.3075 |
49.089 |
210.510 |
0.8554 |
- |
| 350 |
118.80 |
2.2432 |
55.698 |
284.450 |
0.8819 |
- |
3.6. Thermodynamic Studies of Adsorption
The three most popular thermodynamic parameters namely, change in Gibb’s free energy (∆G), change in enthalpy (∆H), and the change in entropy (∆S) were evaluated using van ‘t Hoff equation, mathematically expressed as (44):
is the distribution coefficient; qe (mg/g) is the amount of adsorbate adsorbed on the sorbent per unit mass. The values of ∆S and ∆H were obtained from the intercept and slope of a linear plot of ln KD against 1/T (Figure not shown), and ∆G was evaluated using (Eq.13):
Endothermic 4C2MP adsorption process with disorderliness was predicted based on the positive values obtained for both ∆H and ∆S, respectively (Table 6).
Furthermore, the negative ∆G values obtained signified a spontaneous process, the higher the negative ∆G value, the more spontaneous the adsorption process. This phenomenon has also been observed in the adsorption of 2,4,6-TCP on activated clay (45). The ∆G values (−6.45 to −6.88) further confirm that the adsorption process of 4C2MP onto PASH-AC is a physical one with the physical adsorption values extending from −20 to 0 kJ/mol while values from −80 to −400 kJ/mol indicate chemical adsorption (46). This phenomenon was also observed in the adsorption of chlorophenols onto other adsorbents (39,47,48).
Table 6.
Thermodynamic Parameters for the Adsorption of 4C2MP onto PASH-AC at Different Temperatures
|
∆H(kJ/mol)
|
∆S (J/mol K)
|
∆G (kJ/mol)
|
|
303 K
|
313 K
|
323 K
|
| 0.03 |
21.47 |
-6.45 |
-6.74 |
-6.88 |
Conclusion
PASH-AC was successfully produced from Prosopis africana seed hulls with its adsorption capacity reported to be surging up with an increase in both initial concentration of 4C2MP as well as adsorption time. Acidic solution was the most favourable route for the adsorption process with the suitability of Langmuir-2, which was reported as the best isotherm model for describing the equilibrium data. Pseudo-second-order model was the best kinetic model for describing the kinetic process of 4C2MP adsorption onto PASH-AC with the mechanism primarily guided by particle diffusion according to the Boyd plot. Endothermic process was also confirmed based on the positive ∆H values observed with its great potential upon comparison with previously reported results available in the literature. The obtained results may be a good base for claiming the effectiveness of PASH-AC produced in tackling pollution problems posed by contaminants belonging to chloroguaicols in the environment.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
References
- Galadima A, Garba ZN, Leke L, Almustapha MN, Adam IK. Domestic water pollution among local communities in Nigeria-causes and consequences. Eur J Sci Res 2011; 52(4):592-603. [ Google Scholar]
- Sun S, Xiao W, You C, Zhou W, Garba ZN, Wang L. Methods for preparing and enhancing photocatalytic activity of basic bismuth nitrate. J Clean Prod 2021; 294:126350. doi: 10.1016/j.jclepro.2021.126350 [Crossref] [ Google Scholar]
- Sun S, Jiang X, Xiao W, Jiang Y, Zhou W, Lawan I. A simple method for construction of Bi2O3/Bi6O6(OH)3(NO3)3·15H2O p–n junction photocatalyst with superior photocatalytic performance. Mater Lett 2020; 276:128199. doi: 10.1016/j.matlet.2020.128199 [Crossref] [ Google Scholar]
- Surip SN, Abdulhameed AS, Garba ZN, Syed-Hassan SSA, Ismail K, Jawad AH. H2SO4-treated Malaysian low rank coal for methylene blue dye decolourization and cod reduction: optimization of adsorption and mechanism study. Surf Interfaces 2020; 21:100641. doi: 10.1016/j.surfin.2020.100641 [Crossref] [ Google Scholar]
- Hui Chuin CT, Sabar S, Haafiz MK, Garba ZN, Hussin MH. The improved adsorbent properties of microcrystalline cellulose from oil palm fronds through immobilization technique. Surf Interfaces 2020; 20:100614. doi: 10.1016/j.surfin.2020.100614 [Crossref] [ Google Scholar]
- Ahmad NL, Zakariyya UZ, Zaharaddeen NG. Rice husk as biosorbent for the adsorption of methylene blue. Sci World J 2019; 14(2):66-70. [ Google Scholar]
- Singh NB, Nagpal G, Agrawal S, Rachna Rachna. Water purification by using Adsorbents: a review. Environ Technol Innov 2018; 11:187-240. doi: 10.1016/j.eti.2018.05.006 [Crossref] [ Google Scholar]
- Armenante PM, Kafkewitz D, Lewandowski GA, Jou CJ. Anaerobic–aerobic treatment of halogenated phenolic compounds. Water Res 1999; 33(3):681-92. doi: 10.1016/s0043-1354(98)00255-3 [Crossref] [ Google Scholar]
- Tan IAW, Ahmad AL, Hameed BH. Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J Hazard Mater 2009; 164(2-3):473-82. doi: 10.1016/j.jhazmat.2008.08.025 [Crossref] [ Google Scholar]
- Adetokun AA, Uba S, Garba ZN. Optimization of adsorption of metal ions from a ternary aqueous solution with activated carbon from Acacia senegal (L) Willd pods using Central Composite Design. J King Saud Univ Sci 2019; 31(4):1452-62. doi: 10.1016/j.jksus.2018.12.007 [Crossref] [ Google Scholar]
- Abdul Rahim A, Garba ZN. Efficient adsorption of 4-chloroguiacol from aqueous solution using optimal activated carbon: equilibrium isotherms and kinetics modeling. J Assn Arab Univ Basic Appl Sci 2016; 21:17-23. doi: 10.1016/j.jaubas.2015.09.001 [Crossref] [ Google Scholar]
- Garba ZN, Hussin MH, Galadima A, Lawan I. Potentials of Canarium schweinfurthii seed shell as a novel precursor for CH3COOK activated carbon: statistical optimization, equilibrium and kinetic studies. Appl Water Sci 2019; 9(2):31. doi: 10.1007/s13201-019-0907-y [Crossref] [ Google Scholar]
- Garba ZN, Zhou W, Lawan I, Xiao W, Zhang M, Wang L. An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: a review. J Environ Manage 2019; 241:59-75. doi: 10.1016/j.jenvman.2019.04.004 [Crossref] [ Google Scholar]
- Hussin MH, Pohan NA, Garba ZN, Kassim MJ, Abdul Rahim A, Brosse N. Physicochemical of microcrystalline cellulose from oil palm fronds as potential methylene blue adsorbents. Int J Biol Macromol 2016; 92:11-9. doi: 10.1016/j.ijbiomac.2016.06.094 [Crossref] [ Google Scholar]
- Xiao W, Garba ZN, Sun S, Lawan I, Wang L, Lin M. Preparation and evaluation of an effective activated carbon from white sugar for the adsorption of rhodamine B dye. J Clean Prod 2020; 253:119989. doi: 10.1016/j.jclepro.2020.119989 [Crossref] [ Google Scholar]
- Xiao W, Jiang X, Liu X, Zhou W, Garba ZN, Lawan I. Adsorption of organic dyes from wastewater by metal-doped porous carbon materials. J Clean Prod 2021; 284:124773. doi: 10.1016/j.jclepro.2020.124773 [Crossref] [ Google Scholar]
- Yang D, Zhao X, Zou X, Zhou Z, Jiang Z. Removing Cr(VI) in water via visible-light photocatalytic reduction over Cr-doped SrTiO3 nanoplates. Chemosphere 2019; 215:586-95. doi: 10.1016/j.chemosphere.2018.10.068 [Crossref] [ Google Scholar]
- Garba ZN, Abdullahi AK, Haruna A, Gana SA. Risk assessment and the adsorptive removal of some pesticides from synthetic wastewater: a review. Beni Suef Univ J Basic Appl Sci 2021; 10(1):19. doi: 10.1186/s43088-021-00109-8 [Crossref] [ Google Scholar]
- Garba ZN, Abdul Rahim A. Adsorption of 4-chlorophenol onto optimum activated carbon from an agricultural waste. Int J Sci Res 2015; 4(5):1931-6. [ Google Scholar]
- Garba ZN, Abdul Rahim A. Evaluation of optimal activated carbon from an agricultural waste for the removal of para-chlorophenol and 2,4-dichlorophenol. Process Saf Environ Prot 2016; 102:54-63. doi: 10.1016/j.psep.2016.02.006 [Crossref] [ Google Scholar]
- García-Araya JF, Beltrán FJ, Álvarez P, Masa FJ. Activated carbon adsorption of some phenolic compounds present in agroindustrial wastewater. Adsorption 2003; 9(2):107-15. doi: 10.1023/a:1024228708675 [Crossref] [ Google Scholar]
- Garba ZN, Shikin FB, Abdul Rahim A. Valuation of activated carbon from waste tea for the removal of a basic dye from aqueous solution. J Chem Eng Chem Res 2015; 2(5):623-33. [ Google Scholar]
- Liu X, Wu Y, Ye H, Chen J, Xiao W, Zhou W, Garba ZN, Lawan I, Wang L, Yuan Z. Modification of sugar-based carbon using lanthanum and cobalt bimetal species for effective adsorption of methyl orange. Environ. Technol. Innov 2021; 23:101769. [ Google Scholar]
- Xiao W, Jiang X, Liu X, Zhou W, Garba ZN, Lawan I. Adsorption of organic dyes from wastewater by metal-doped porous carbon materials. J Clean Prod 2021; 284:124773. doi: 10.1016/j.jclepro.2020.124773 [Crossref] [ Google Scholar]
- Abdul Rahim A, Garba ZN. Optimization of preparation conditions for activated carbon from Prosopis africana seed hulls using response surface methodology. Desalin Water Treat 2016; 57(38):17985-94. doi: 10.1080/19443994.2015.1086695 [Crossref] [ Google Scholar]
- Garba ZN, Abdul Rahim A. Process optimization of K2C2O4-activated carbon from Prosopis africana seed hulls using response surface methodology. J Anal Appl Pyrolysis 2014; 107:306-12. doi: 10.1016/j.jaap.2014.03.016 [Crossref] [ Google Scholar]
- Garba ZN, Abdul Rahim A. Potentials of Prosopis africana Seed Hulls (PASH) as Precursor for Activated Carbon Preparation Using Sodium Acetate as Activating Agent: Optimization Using Response Surface Methodology. In: Int Conf Chem Environ Sci Res ; 2014; Penang, Malaysia.
- Tan IAW, Ahmad AL, Hameed BH. Preparation of activated carbon from coconut husk: optimization study on removal of 2,4,6-trichlorophenol using response surface methodology. J Hazard Mater 2008; 153(1-2):709-17. doi: 10.1016/j.jhazmat.2007.09.014 [Crossref] [ Google Scholar]
- Garba ZN, Abdul Rahim A, Hamza SA. Potential of Borassus aethiopum shells as precursor for activated carbon preparation by physico-chemical activation; optimization, equilibrium and kinetic studies. J Environ Chem Eng 2014; 2(3):1423-33. doi: 10.1016/j.jece.2014.07.010 [Crossref] [ Google Scholar]
- Hamad BK, Noor AM, Afida AR, Mohd Asri MN. High removal of 4-chloroguaiacol by high surface area of oil palm shell-activated carbon activated with NaOH from aqueous solution. Desalination 2010; 257(1-3):1-7. doi: 10.1016/j.desal.2010.03.007 [Crossref] [ Google Scholar]
- Hameed BH, Tan IAW, Ahmad AL. Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chem Eng J 2008; 144(2):235-44. doi: 10.1016/j.cej.2008.01.028 [Crossref] [ Google Scholar]
- Hamad BK, Ahmad MN, Abdul Rahim A. Removal of 4-chloro-2-methoxy phenol by adsorption from aqueous solution using oil palm shell carbon activated by K2CO3. J Phys Sci 2011; 22(1):41-58. [ Google Scholar]
- Langmuir I. The constitution and fundamental properties of solids and liquids Part I Solids. J Am Chem Soc 1916; 38(11):2221-95. doi: 10.1021/ja02268a002 [Crossref] [ Google Scholar]
- Baccar R, Blánquez P, Bouzid J, Feki M, Attiya H, Sarrà M. Modeling of adsorption isotherms and kinetics of a tannery dye onto an activated carbon prepared from an agricultural by-product. Fuel Process Technol 2013; 106:408-15. doi: 10.1016/j.fuproc.2012.09.006 [Crossref] [ Google Scholar]
- Sadaf S, Bhatti HN, Nausheen S, Amin M. Removal of Cr(VI) from wastewater using acid-washed zero-valent iron catalyzed by polyoxometalate under acid conditions: Efficacy, reaction mechanism and influencing factors. J Taiwan Inst Chem Eng 2015; 47:160-70. [ Google Scholar]
- Freundlich HM. Over the adsorption in solution. J Phys Chem 1906; 57:385-470. [ Google Scholar]
- Temkin MJ, Pyzhev V. Recent modifications to Langmuir isotherms. Acta Physiochim 1940; 12:217-22. [ Google Scholar]
- Hameed BH, Chin LH, Rengaraj S. Adsorption of 4-chlorophenol onto activated carbon prepared from rattan sawdust. Desalination 2008; 225(1-3):185-98. doi: 10.1016/j.desal.2007.04.095 [Crossref] [ Google Scholar]
- Ren L, Zhang J, Li Y, Zhang C. Preparation and evaluation of cattail fiber-based activated carbon for 2,4-dichlorophenol and 2,4,6-trichlorophenol removal. Chem Eng J 2011; 168(2):553-61. doi: 10.1016/j.cej.2011.01.021 [Crossref] [ Google Scholar]
- Wang SL, Tzou YM, Lu YH, Sheng G. Removal of 3-chlorophenol from water using rice-straw-based carbon. J Hazard Mater 2007; 147(1-3):313-8. doi: 10.1016/j.jhazmat.2007.01.031 [Crossref] [ Google Scholar]
- Lagergren S, Svenska BK. On the theory of so-called adsorption of dissolved substances. The Royal Swedish Academy of Sciences Document 1898; 24:1-13. [ Google Scholar]
- Wang L, Zhang J, Zhao R, Li C, Li Y, Zhang C. Adsorption of basic dyes on activated carbon prepared from Polygonum orientale Linn: equilibrium, kinetic and thermodynamic studies. Desalination 2010; 254(1-3):68-74. doi: 10.1016/j.desal.2009.12.012 [Crossref] [ Google Scholar]
- Khaled A, Nemr AE, El-Sikaily A, Abdelwahab O. Removal of Direct N Blue-106 from artificial textile dye effluent using activated carbon from orange peel: adsorption isotherm and kinetic studies. J Hazard Mater 2009; 165(1-3):100-10. doi: 10.1016/j.jhazmat.2008.09.122 [Crossref] [ Google Scholar]
- Slimani R, El Ouahabi I, Abidi F, El Haddad M, Regti A, Laamari MR. Calcined eggshells as a new biosorbent to remove basic dye from aqueous solutions: thermodynamics, kinetics, isotherms and error analysis. J Taiwan Inst Chem Eng 2014; 45(4):1578-87. doi: 10.1016/j.jtice.2013.10.009 [Crossref] [ Google Scholar]
- Hameed BH. Equilibrium and kinetics studies of 2,4,6-trichlorophenol adsorption onto activated clay. Colloids Surf A Physicochem Eng Asp 2007; 307(1):45-52. doi: 10.1016/j.colsurfa.2007.05.002 [Crossref] [ Google Scholar]
- Li Q, Yue QY, Su Y, Gao BY, Sun HJ. Equilibrium, thermodynamics and process design to minimize adsorbent amount for the adsorption of acid dyes onto cationic polymer-loaded bentonite. Chem Eng J 2010; 158(3):489-97. doi: 10.1016/j.cej.2010.01.033 [Crossref] [ Google Scholar]
- Agarry SE, Owabor CN, Ajani AO. Modified plantain peel as cellulose-based low-cost adsorbent for the removal of 2,6-dichlorophenol from aqueous solution: adsorption isotherms, kinetic modeling, and thermodynamic studies. Chem Eng Commun 2013; 200(8):1121-47. doi: 10.1080/00986445.2012.740534 [Crossref] [ Google Scholar]
- Sathishkumar M, Binupriya AR, Kavitha D, Yun SE. Kinetic and isothermal studies on liquid-phase adsorption of 2,4-dichlorophenol by palm pith carbon. Bioresour Technol 2007; 98(4):866-73. doi: 10.1016/j.biortech.2006.03.002 [Crossref] [ Google Scholar]