Avicenna J Environ Health Eng. 8(1):28-32.
doi: 10.34172/ajehe.2021.05
Original Article
Evaluation of the Antibacterial Potential of Essential Oil and Extract of Apium graveolens L. as an Environmentally Friendly Technology Against Helicobacter pylori
Hanie Ahmadpour Kacho 1  , Mostafa leili 2, Mojtaba Masoumi 3, Pezhman Farhadi 3, *
, Mostafa leili 2, Mojtaba Masoumi 3, Pezhman Farhadi 3, * 
Author information:
1Student Research Committee, Department of Environmental Health Engineering, Hamadan University of Medical Sciences, Hamadan, Iran
2Department of Environmental Health Engineering, School of Public Health, Hamadan University of Medical Sciences, Hamadan, Iran
3Department of Chemical Engineering, Ayatollah Amoli Branch, Islamic Azad University, Amol, Iran
Abstract
The hygiene hypothesis links the environmental and microbial exposure in early life to the prevalence of atopy, allergy, and asthma. Helicobacter pylori infection is typically acquired in childhood and the acquisition is associated with poor household hygiene. In this study, the current knowledge on the activity of essential oils and extract of Apium graveolens L. (celery seeds) and their derivatives against H. pylori was reviewed. Celery seeds were prepared at the Pharmaceutical Farm (Babol, Mazandaran) of Iran. The essential oils were extracted by a Clevenger approach and analyzed using GC-MS, and maceration method was used to prepare the extract. H. pylori bacteria were isolated by the cultivation of gastric biopsy removed from the patient who had gastric ulcer. The antibacterial activities of both essential oils and extract against H. pylori were evaluated by agar dilution method, and the corresponding MIC (minimum inhibitory concentration) and MBC (minimum bactericidal concentration) values were determined for each sample. The results showed that the main components in celery seed essential oils were α-pinene (20.25%), β-pinene (16.62%), and Sabinene (7.81%). Among different samples, essential oils of celery seed exhibited better effect on H. pylori with MIC value of 1.56 mg/mL and MBC value of 3.12 mg/mL. Methanol and water extracts of celery seed showed MIC values of 0.38 mg/mL and 0.78 mg/mL and MBC values of 1.56 mg/mL and 6.25 mg/ mL, respectively (P≤0.005). The results of this study indicated that all the samples had antibacterial effect against H. pylori due to antibacterial components.
Keywords: Essential oil, Extract, Celery seed, Helicobacter pylori, Resistance
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
Helicobacter pylori is considered the main etiological agent of gastritis, ulcers, and gastric carcinoma. It colonizes human gastric mucosa quite efficiently (1). In developing countries, many communities lack access to a reliable source of clean water or sanitation services. Moreover, those communities find themselves having no other choice but to depend on the surrounding sources of continuously flowing water, such as nearby rivers and streams as their sole everyday water source. On the other hand, isolated communities living in low-populated deserted geographical areas, located hundreds of miles away from a nearby river branch or stream, are obliged to rely on municipal water wells as their main supply for drinking and irrigation. An alarmingly rising number of those individuals suffer from numerous gastrointestinal tract-related problems, some of which can be directly linked to Helicobacter pylori infection, which can result in chronic infection and even cancer (2,3). H. pylori infection is extremely common worldwide, and more than two-thirds of the world’s population is infected with this organism. H. pylori is recognized as the major etiological factor in chronic active type B gastritis, gastric ulcers, and gastric cancer (4). H. pylori pathogenesis is mediated by a complex interplay between various bacterial virulence factors, host genetic predisposition, and environmental factors. H. pylori can enter a viable but non-culturable state under adverse conditions, such as those present in faeces, under fully aerobic conditions and in low water activity environments (5,6). H. pylori is also known as one of the most genetically diverse bacterial species that presents various virulence genotypes responsible for different gastric diseases (7-9). Present treatments for H. pylori infections are based on the combination of a proton pump inhibitor and two antibiotics (triple therapy) (4). Eradicating H. pylori is the most promising strategy at present, as it both reduces morbidity of H. pylori-related disorders and decreases the incidence of gastric cancer (10-12). Triple therapy containing a proton pump inhibitor and two antibiotics has been the most widely recommended first-line therapy for eradicating H. pylori for a long time (13). Antibiotic resistance and non-compliance due to secondary effects are the major causes of eradication treatment failure. There are several ways to decrease treatment failure such as finding new and more potent drugs to kill the bacteria, developing a vaccine to stimulate the host immune defense, or developing new nutritional approaches to the management of the infection (4). In developing countries, since the application of antibiotics is still under poor management as a whole, there is a growing need for finding new anti-H. pylori agents that can hopefully eradicate the invasion and presence of survived H. pylori strains to prevent relapse of gastric ulcer. The interrelationships between society and nature and the importance of environmental health to human health have recently become widely acknowledged. Some plants are present in the environment and nature, and many of them have a significant role to play in the treatment of diseases. Therefore, a considerable variety of studies involving tests for medicinal plants showing antimicrobial activity and discrepant susceptibility test results are available due to variations in the methods and conditions used for its susceptibility testing. Hence, numerous studies have focused on the eradication of H. pylori infection using traditional herbal medicines. Garlic and Pteleopsis extracts exhibited weak and modest anti-H. pylori activity, respectively (14,15). Apium graveolens extract was screened for anti-H. pylori activity (16). Satureja bachtiarica Bunge essential oil possesses moderate anti-H. pylori activity (17). The anti-H. pylori activities of Crocus sativus L., Agrimonia eupatoriaL., and Fragaria vescawere also examined (18,19). Celery (Apium graveolens L.) is one of the aromatic vegetables that is consumed daily in most parts of the world. A previous study has reported that celery seeds and other parts of celery are known as appropriate herbal medicines (20). Several studies (21,22) revealed that some traditional herbal medicines are efficient against gastrointestinal diseases, including chronic gastritis and peptic ulcer disease, a major outcome of H. pylori and anti-inflammatory activities. The present study was conducted to evaluate the H. pylori activity of celery seeds to identify the potential sources for the synthesis of new drugs against H. pylori. In this study, essential oil and methanol and water extract were examined and screened for their anti-H. pylori activity according to their minimum inhibitory concentration (MIC).
2. Materials and Methods
2.1. Plant Material
Fresh celery seeds were harvested from a herb farm in Babol city, Mazandaran province, in the north of Iran in august 2019. All chemicals used in this experiment were purchased from local dealers of Sigma (USA) and Merck (Germany). The chemical components of celery seed essential oil were obtained using an AGILENT gas chromatograph (AGILENT 7890, CA, USA).
2 .2. Preparation of Extracts and Essential Oil
Celery seeds were washed with sterile distilled water and air-dried in a dark place for 3 days. Then, the dried celery seeds were powdered by an electrical mill. Extraction was performed by adding 500 mL of methanol and water solvent to 50 g of each sample, and the samples were placed in an incubator-shaker at room temperature for 72 hours. The mixture of methanol and water solvent was filtered through the Whatman filter paper under vacuum pump. The coarse ingredients were separated and the mixture was evaporated for removing the solvent using a rotary evaporator. The filtrates were dried in an oven at 40°C and stored at 4°C until further use. Following the extraction of essential oil, 100 g of powdered seeds was added to 1000 mL of sterile distilled water on top of the heater at 100°C for 4 hours in a Clevenger-type apparatus. The essential oil of celery seeds was extracted from the bottom of glass distillation column, dried with sodium sulphate for removing water, and stored in a fridge in a dark place until use.
2.3. Helicobacter pylori and Cell Culture Conditions
This study was performed on H. pylori strains collected in the Department of Microbiology, Babol University of Medical Sciences, Babol, Iran. The isolated strains were collected from patients with gastric ulcer that were referred to Ayatollah Rouhani Hospital (Babol, Mazandaran). The urease positive biopsies were transferred to Research Laboratory using the transport culture medium containing 10% normal saline. The diagnosis of H. pylori strain was performed using several tests such as morphological test, Gram staining, catalase test, and oxidase test. These bacteria were cultured on Brucella agar medium containing sheep blood (7%) and selective supplement containing different antibiotics such as vancomycin (0/01 g/L), polymyxin B (0.025 g/L), and trimethoprim (0.005 g/L) under microaerophilic conditions ( 5% O2, 10% CO2, 85% N2 ) was used for the incubation of H. pylori for 3-7 days at 37°C. Eventually, the diagnosis of H. pylori strain was performed using several tests such as morphological test, Gram staining, catalase test and oxidase test as well as biochemical tests.
2.4. Determination of Helicobacter pylori Activity
The H. pylori activities of essential oil and extracts of celery seeds were tested using the agar dilution method. Different concentration of water and methanol extracts of celery seeds (500, 250, 125, 62.5, 31.25, 15.62, 7.81 mg) and essential oil of celery seed (10, 5, 2.5, 1.25, 0.625 mg) were determined. Different concentrations of extract were added to each medium plate containing 20 mL of H. pylori selective supplement that was described above. It was inoculated with suspension of the fresh H. pylori (Approximately 1×108, turbidity compared with 0.5 McFarland). Plates of bacteria suspension without essential oil, extracts, and antibiotics were used as control samples. All plates were incubated at 37°C with specific condition (5% O2, 10% CO2 and 85% N2) in moist atmosphere for 3-7 days. MIC was determined as the lowest concentration that growth of H. pylori could be visible. Overall, it was compared with bacterial growth in the plates of control samples. MBC is the minimal bactericidal concentration necessary to kill and inhibit growth of bacteria. Therefore, we used MIC values to introduce susceptibility of H. pylori, as an infectious agent responsible for gastric ulcer disease, against natural herbal medicines.
3. Results and Discussion
To determine if celery seeds oil or extract might represent a natural product with antimicrobial activity against H. pylori, we investigated the antimicrobial activity of essential oil and extracts of celery seeds and found that they had bactericidal activity against H. pylori. The activity depended on the concentration and type of solvent used in the extraction process. In our study, methanol extract has shown higher anti-microbial activity against H. pylori strains isolated from clinical cases of gastrointestinal disorders than essential oil and water extract. The MIC and MBC results indicate that the extracts and essential oil compounds have significant but differing effects against H. pylori growth (Table 1). Methanol extract showed the highest activity (MIC: 0.38 mg/mL and MBC: 0.781 mg/mL), followed by essential oil (MIC: 1.56 mg/mL and MBC: 3.12 mg/mL) and water extract (MIC: 1.56 mg/mL and MBC: 6.25 mg/mL). The antibacterial activity of herbal compounds extracted from plants depends upon the type of solvent used for extraction. Seed extracts of A. graveolens showed a broad spectrum of antibacterial activity on the tested microorganisms. It was clear that methanol extract showed the highest activity against bacterial strains. This study corroborates the findings of a previous study (23) which found that the methanol extract of Euphorbiaceae plants showed the highest inhibitory activity against urinary tract pathogens including E. coli, K. pneumonia,and P. aeruginosa. Antibacterial and anti-H. pylori activities of celery seeds were reported in a study by Zhou et al (24). Concentrations of a novel component from celery seeds with minimum inhibitory activities and bactericidal properties were reported to be 3.15 µg/mL and 6.25-12.5 µg/mL, respectively. Essential oils obtained from plants are aromatic in nature because of a mixture of multifarious chemical substances that belong to different chemical families, including terpenes, aldehydes, alcohols, esters, phenolic, ethers, and ketones (25,26). It was revealed that the combination of some particular oils produced synergism as a result of the combined activities of two or more constituents of essential oils. Because pathogens cannot easily acquire resistance to multiple components of two or more essential oils, it can be a good alternative to antibiotics that H. pylori strains are resistant to (27). In this study, the essential oil and various organic extracts of celery seeds exhibited potential activity against H. pylori. The results of GC–MS analysis of celery seed essential oil are given in Table 2 and Figure 1. A total of 28 volatile compounds were identified in essential oils originating from celery seeds. The identified compounds represent 54.86% total essential oil components. The dominant components of essential oil were α-pinene (20.25%), β-pinene (16.62%), Sabinene (7.81%). In our opinion, major components of the oil, α-pinene (20.25%), β-pinene (16.62%), and Sabinene (7.81%), have key roles in their antibacterial activities. Antibacterial activities of these compounds have been reported by others (28-30). Additionally, the antibacterial activities of individual components of essential oils such as α-pinene and β-pinene have been reported in previous studies (31,32). On the other hand, the components in lower amounts such as myrcene, limonene, cymene, and Terpinolene also contributed to the antibacterial activity of the oils (33,34). It is also possible that the minor components might be involved in some type of synergism with the other active compounds (35).
Table 1.
MICs and MBCs of Extracts and Essential Oil against Helicobacter Pylori Isolates
|
Samples
|
MIC (mg/mL)
|
MBC (mg/mL)
|
| Methanol extract of celery seed |
0.38 |
0.78 |
| Water extract of celery seed |
1.56 |
6.25 |
| Essential oil of celery seed |
1.56 |
3.12 |
Table 2.
GC-MS Compound Name, Retention Time and Peak Value Obtained for the Essential Oil of Celery Seed
|
R.T
|
Compounds
|
Percentage of total oil (%)
|
| 6.124 |
a-thujene |
0.312 |
| 6.804 |
a-pinene |
20.25 |
| 7.592 |
Comphene |
0.54 |
| 9.986 |
b-Pinene |
16.625 |
| 10.748 |
Myrcene |
1.52 |
| 11.748 |
a-Phellandrene |
0.546 |
| 12.45 |
a-Terpinene |
0.293 |
| 13.203 |
Limonene |
1.36 |
| 13.782 |
Sabinene |
7.815 |
| 14.126 |
cymene |
0.752 |
| 14.406 |
b-Ocimene |
0.093 |
| 15.165 |
Gamma-terpinene |
0.72 |
| 16.438 |
Terpinolene |
0.456 |
| 17.974 |
Isopropenyl toluene |
0.434 |
| 21.09 |
Moslene |
0.3 |
| 21.893 |
Pinene |
0.32 |
| 22.513 |
Paracymenyl |
0.155 |
| 22.932 |
Benihinal |
0.131 |
| 24.13 |
Thymol |
0.1 |
| 24.615 |
Cuminic aldehyde |
0.097 |
| 24.826 |
Langipinene |
0.233 |
| 25.384 |
m-Thymol |
0.192 |
| 25.592 |
Gamma-cadinene |
0.36 |
| 25.740 |
Cumin alcohol |
0.127 |
| 25.837 |
Isoestragole |
0.112 |
| 27.236 |
Caryophyllene |
0.38 |
| 27.989 |
b-Farnesene |
0.555 |
| 28.463 |
Germacrene |
0.09 |
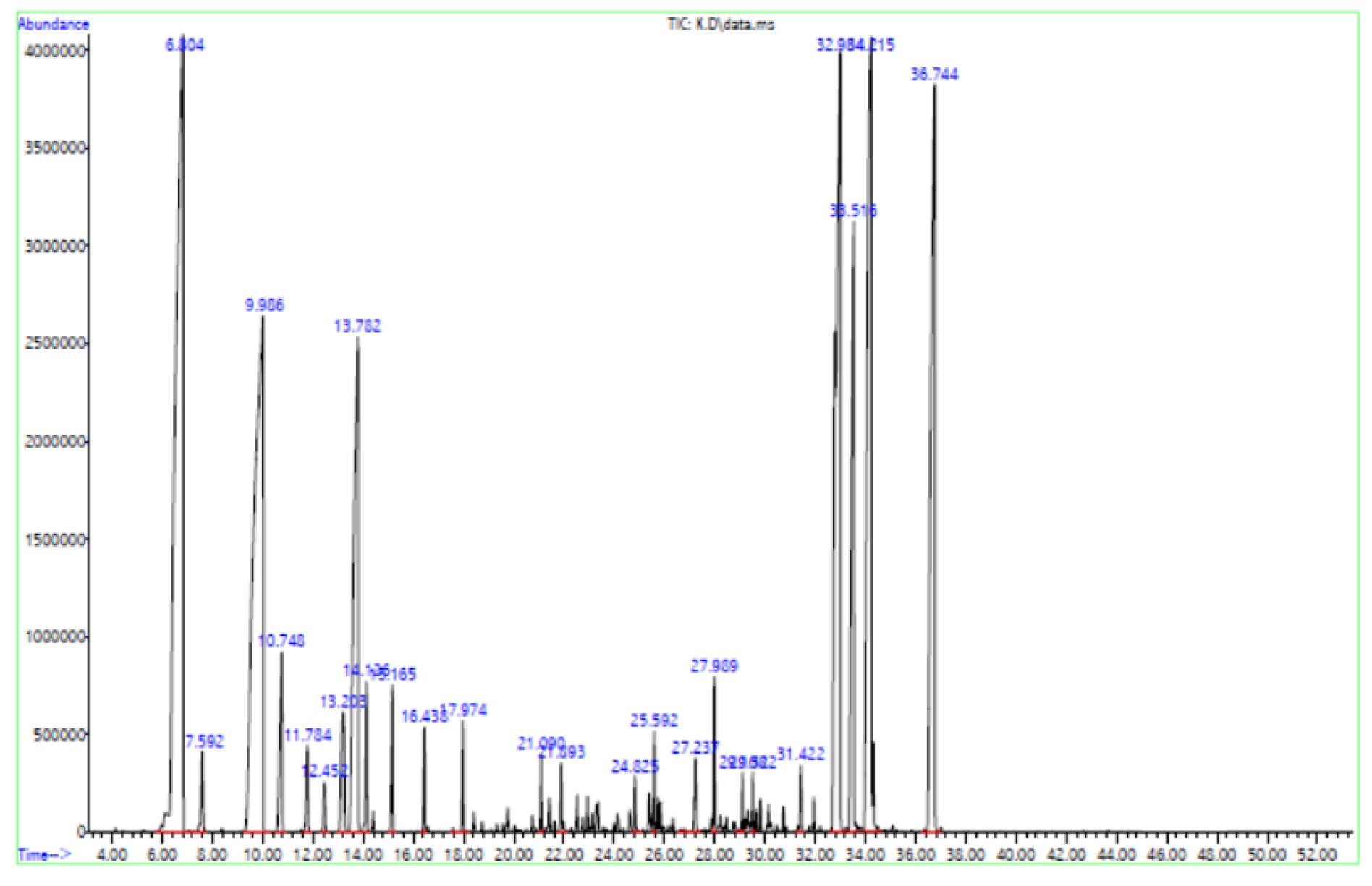
Figure 1.
GC-MS Analysis of the Respective Compounds of Essential Oil of Celery Seed.
.
GC-MS Analysis of the Respective Compounds of Essential Oil of Celery Seed.
4. Conclusion
Helicobacter pylori infection most likely acts as a weak surrogate for the presence of poor hygiene. An increased prevalence of infection has been associated with increased consumption of food from street vendors, supporting the role of food prepared under unhygienic conditions as a probable mechanism of transmission. Antibiotic resistance is an increasing problem as its use has become more common in recent years. Although celery seed has been used as a crude drug in Iranian folk medicine for more than 2000 years, we observed that H. pylori strain have not developed resistance to flavonoids and phenolic acid contents of the celery seeds. The methanol extract showed inhibitory effects on the growth of H. pylori at much higher concentrations; however, the results suggest that these extracts and essential oil have a moderate antibacterial activity against H. pylori. Therefore, this compound may be useful as a lead compound in the development of a new class of anti-H. pylori agents.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
Acknowledgements
The authors are highly thankful to Dr. Ramazan Rajabnia (Department of Microbiology and Immunology, Babol University of Medical Sciences) and Dr. Javad Shokri-Shirvani (Department of Internal Medicine, Babol University of Medical Sciences). Additionally, the authors are thankful to Experts of the Microbiology Laboratory (Department of Microbiology and Immunology, Babol University of Medical Sciences) and Laboratory of Agricultural and Food Industries (Ayatollah Amoli Branch, Islamic Azad University).
References
- Venegas A, Touma JH, Bravo J, Perez-Perez G. Progress in use of natural products and their active components against Helicobacter pylori. Adv Microbiol 2016; 6(14):1091-129. doi: 10.4236/aim.2016.614101 [Crossref] [ Google Scholar]
- Ghanbari F, Vaez H, Taheri RA, Sahebkar A, Behshod P, Khademi F. Helicobacter pylori in water, vegetables and foods of animal origin: a systematic review and meta-analysis on the prevalence, antibiotic resistance and genotype status in Iran. Gene Rep 2020; 21:100913. doi: 10.1016/j.genrep.2020.100913 [Crossref] [ Google Scholar]
- Shiota S, Reddy R, Alsarraj A, El-Serag HB, Graham DY. Antibiotic resistance of Helicobacter pylori among male United States veterans. Clin Gastroenterol Hepatol 2015; 13(9):1616-24. doi: 10.1016/j.cgh.2015.02.005 [Crossref] [ Google Scholar]
- Bergonzelli GE, Donnicola D, Porta N, Corthésy-Theulaz IE. Essential oils as components of a diet-based approach to management of Helicobacter infection. Antimicrob Agents Chemother 2003; 47(10):3240-6. doi: 10.1128/aac.47.10.3240-3246.2003 [Crossref] [ Google Scholar]
- Lima VP, Silva-Fernandes IJ, Alves MK, Rabenhorst SH. Prevalence of Helicobacter pylori genotypes (vacA, cagA, cagE and virB11) in gastric cancer in Brazilian’s patients: an association with histopathological parameters. Cancer Epidemiol 2011; 35(5):e32-7. doi: 10.1016/j.canep.2011.02.017 [Crossref] [ Google Scholar]
- Bugaytsova JA, Björnham O, Chernov YA, Gideonsson P, Henriksson S, Mendez M. Helicobacter pylori adapts to chronic infection and gastric disease via pH-responsive BabA-mediated adherence. Cell Host Microbe 2017; 21(3):376-89. doi: 10.1016/j.chom.2017.02.013 [Crossref] [ Google Scholar]
- Taremi M, Soltan Dallal MM, Gachkar L, MoezArdalan S, Zolfagharian K, Reza Zali M. Prevalence and antimicrobial resistance of Campylobacter isolated from retail raw chicken and beef meat, Tehran, Iran. Int J Food Microbiol 2006; 108(3):401-3. doi: 10.1016/j.ijfoodmicro.2005.12.010 [Crossref] [ Google Scholar]
- Yadegar A, Alebouyeh M, Lawson AJ, Mirzaei T, Nazemalhosseini Mojarad E, Zali MR. Differentiation of non-pylori Helicobacter species based on PCR-restriction fragment length polymorphism of the 23S rRNA gene. World J Microbiol Biotechnol 2014; 30(6):1909-17. doi: 10.1007/s11274-014-1615-2 [Crossref] [ Google Scholar]
- Yadegar A, Alebouyeh M, Zali MR. Analysis of the intactness of Helicobacter pylori cag pathogenicity island in Iranian strains by a new PCR-based strategy and its relationship with virulence genotypes and EPIYA motifs. Infect Genet Evol 2015; 35:19-26. doi: 10.1016/j.meegid.2015.07.026 [Crossref] [ Google Scholar]
- Liu Q, Meng X, Li Y, Zhao CN, Tang GY, Li S. Natural products for the prevention and management of Helicobacter pylori infection. Compr Rev Food Sci Food Saf 2018; 17(4):937-52. doi: 10.1111/1541-4337.12355 [Crossref] [ Google Scholar]
- Parreira P, Fátima Duarte M, Reis CA, Martins MC. Helicobacter pylori infection: a brief overview on alternative natural treatments to conventional therapy. Crit Rev Microbiol 2016; 42(1):94-105. doi: 10.3109/1040841x.2014.892055 [Crossref] [ Google Scholar]
- Talebi Bezmin Abadi A. Strategies used by Helicobacter pylori to establish persistent infection. World J Gastroenterol 2017; 23(16):2870-82. doi: 10.3748/wjg.v23.i16.2870 [Crossref] [ Google Scholar]
- Feng L, Wen MY, Zhu YJ, Men RT, Yang L. Sequential therapy or standard triple therapy for Helicobacter pylori infection: an updated systematic review. Am J Ther 2016; 23(3):e880-93. doi: 10.1097/mjt.0000000000000191 [Crossref] [ Google Scholar]
- Cellini L, Di Campli E, Masulli M, Di Bartolomeo S, Allocati N. Inhibition of Helicobacter pylori by garlic extract (Allium sativum). FEMS Immunol Med Microbiol 1996; 13(4):273-7. doi: 10.1111/j.1574-695X.1996.tb00251.x [Crossref] [ Google Scholar]
- Germanò MP, Sanogo R, Guglielmo M, De Pasquale R, Crisafi G, Bisignano G. Effects of Pteleopsis suberosa extracts on experimental gastric ulcers and Helicobacter pylori growth. J Ethnopharmacol 1998; 59(3):167-72. doi: 10.1016/s0378-8741(97)00109-8 [Crossref] [ Google Scholar]
- Falsafi T, Moradi P, Mahboubi M, Rahimi E, Momtaz H, Hamedi B. Chemical composition and anti-Helicobacter pylori effect of Satureja bachtiarica Bunge essential oil. Phytomedicine 2015; 22(1):173-7. doi: 10.1016/j.phymed.2014.11.012 [Crossref] [ Google Scholar]
- Altameme HJ, Hameed IH, Hamza LF. Anethum graveolens: physicochemical properties, medicinal uses, antimicrobial effects, antioxidant effect, anti-inflammatory and analgesic effects: a review. Int J Pharm Qual Assur 2017; 8(3):88-91. doi: 10.25258/ijpqa.v8i03.9569 [Crossref] [ Google Scholar]
- De Monte C, Bizzarri B, Gidaro MC, Carradori S, Mollica A, Luisi G. Bioactive compounds of Crocus sativus L and their semi-synthetic derivatives as promising anti-Helicobacter pylori, anti-malarial and anti-leishmanial agents. J Enzyme Inhib Med Chem 2015; 30(6):1027-33. doi: 10.3109/14756366.2014.1001755 [Crossref] [ Google Scholar]
- Cardoso O, Donato MM, Luxo C, Almeida N, Liberal J, Figueirinha A. Anti-Helicobacter pylori potential of Agrimonia eupatoria L and Fragaria vesca. J Funct Foods 2018; 44:299-303. doi: 10.1016/j.jff.2018.03.027 [Crossref] [ Google Scholar]
- Tashakori-Sabzevar F, Razavi BM, Imenshahidi M, Daneshmandi M, Fatehi H, Entezari Sarkarizi Y. Evaluation of mechanism for antihypertensive and vasorelaxant effects of hexanic and hydroalcoholic extracts of celery seed in normotensive and hypertensive rats. Rev Bras Farmacogn 2016; 26(5):619-26. doi: 10.1016/j.bjp.2016.05.012 [Crossref] [ Google Scholar]
- Lu B, Chen MT, Fan YH, Liu Y, Meng LN. Effects of Helicobacter pylori eradication on atrophic gastritis and intestinal metaplasia: a 3-year follow-up study. World J Gastroenterol 2005; 11(41):6518-20. doi: 10.3748/wjg.v11.i41.6518 [Crossref] [ Google Scholar]
- Wang Y. Clinical observation on the method of supplementing qi, clearing away heat and promoting blood circulation for treating 53 cases of gastritis related to pyrolic Helicobacterium. J Tradit Chin Med 2003; 23(2):83-6. [ Google Scholar]
- Ramesh N, Sumathi CS, Balasubramanian V, Palaniappan KR, Kannan VR. Urinary tract infection and antimicrobial susceptibility pattern of extended spectrum of beta lactamase producing clinical isolates. Adv Biol Res 2008; 2(5-6):78-82. [ Google Scholar]
- Zhou Y, Taylor B, Smith TJ, Liu ZP, Clench M, Davies NW. A novel compound from celery seed with a bactericidal effect against Helicobacter pylori. J Pharm Pharmacol 2009; 61(8):1067-77. doi: 10.1211/jpp/61.08.0011 [Crossref] [ Google Scholar]
- Akthar MS, Degaga B, Azam T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: a review. Issues Biol Sci Pharm Res 2014; 2(1):1-7. [ Google Scholar]
- Degenhardt J, Köllner TG, Gershenzon J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009; 70(15-16):1621-37. doi: 10.1016/j.phytochem.2009.07.030 [Crossref] [ Google Scholar]
- Stević T, Berić T, Šavikin K, Soković M, Gođevac D, Dimkić I. Antifungal activity of selected essential oils against fungi isolated from medicinal plant. Ind Crops Prod 2014; 55:116-22. doi: 10.1016/j.indcrop.2014.02.011 [Crossref] [ Google Scholar]
- Khalili S, Khoshandam B, Jahanshahi M. A comparative study of CO2 and CH4 adsorption using activated carbon prepared from pine cone by phosphoric acid activation. Korean J Chem Eng 2016; 33(10):2943-52. doi: 10.1007/s11814-016-0138-y [Crossref] [ Google Scholar]
- Khalil N, Ashour M, Fikry S, Singab AN, Salama O. Chemical composition and antimicrobial activity of the essential oils of selected Apiaceous fruits. Futur J Pharm Sci 2018; 4(1):88-92. doi: 10.1016/j.fjps.2017.10.004 [Crossref] [ Google Scholar]
- Kamdem MS, Sameza ML, Dongmo PM, Boyom FF, Bakargna-Via I, Fokou JB. Antiradical, anti-inflammatory and antifungal activities of essential oils of two aromatic plants: Apium graveolens (Apiaceae) and Thymus vulgaris (Lamiaceae). J Life Sci 2015; 9(23):51-64. [ Google Scholar]
- Saeed M, Yasmin I, Khan MI, Nadeem M, Shabbir MA, Azam M. Herbs and spices as a potential antimicrobial agents for food application. Pak J Food Sci 2016; 26(3):153-60. [ Google Scholar]
- de Almeida Pinheiro M, Magalhães RM, Torres DM, Cavalcante RC, Mota FS, Oliveira Coelho EM. Gastroprotective effect of alpha-pinene and its correlation with antiulcerogenic activity of essential oils obtained from Hyptis species. Pharmacogn Mag 2015; 11(41):123-30. doi: 10.4103/0973-1296.149725 [Crossref] [ Google Scholar]
- Salehi P, Sonboli A, Asghari B. Chemical composition of the essential oil of Stachys acerosa and its antibacterial and antioxidant activities. Chem Nat Compd 2007; 43(3):339-41. doi: 10.1007/s10600-007-0126-x [Crossref] [ Google Scholar]
- Deba F, Xuan TD, Yasuda M, Tawata S. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn var Radiata. Food Control 2008; 19(4):346-52. doi: 10.1016/j.foodcont.2007.04.011 [Crossref] [ Google Scholar]
- Marino M, Bersani C, Comi G. Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae. Int J Food Microbiol 2001; 67(3):187-95. doi: 10.1016/s0168-1605(01)00447-0 [Crossref] [ Google Scholar]