Avicenna J Environ Health Eng. 8(1):22-27.
doi: 10.34172/ajehe.2021.04
Original Article
Evaluation of Cefixime Toxicity Treated With Sono-electro-Fenton Process by Bioassay Using Microorganisms
Kamal Hasani 1, 2  , Mina Moradi 1, 2, Abdollah Dargahi 3, *
, Mina Moradi 1, 2, Abdollah Dargahi 3, *  , Mehdi Vosoughi 2, 3, *
, Mehdi Vosoughi 2, 3, *
Author information:
1Student Research Committee, Ardabil University of Medical Sciences, Ardabil, Iran
2Department of Environmental Health Engineering, School of Public Health, Ardabil University of Medical Sciences, Ardabil, Iran
3Social Determinants of Health Research Center, Ardabil University of Medical Sciences, Ardabil, Iran
Abstract
The aim of this study was to determine the toxicity of cefixime in the inlet solution and effluent treated with the sono-electro-Fenton process using standard strains of microorganisms. This research was performed as an experimental study, which was conducted on a laboratory scale. The standard strains of Escherichia coli (gram-negative) and Staphylococcus aureus (gram-positive) were used for bioassay. First, the stock solution of 1000 mg/L containing Cefixime was prepared, and for each bacterium (gram-positive and gram-negative), 5 samples from the inlet solution of the reactor and 5 samples from effluent treated with the sono-electro-Fenton process were collected under optimal conditions. Finally, each sample was transferred to 10 mL of sterile lactose broth, and a loop of E. coli or S. aureus was dissolved in each sample. Toxicity changes were investigated by calculating the percentage of growth inhibition. The results showed that after 10 hours, the growth rate of both bacteria in the control and the effluent samples was higher, while the growth of bacteria in the inlet solution was lower and had higher toxicity. Based on the results of the study, the toxicity rate for E. coli was reduced from 70% in the inlet solution to 9.3% in the effluent (86.7% reduction in toxicity), and in the case of S. aureus, it was diminished from 25.3% in the inlet solution to 7% in the effluent (72.3% reduction in toxicity) after 10 hours. Based on the results of the present study, bioassay using microorganisms is an effective and useful method to study changes in the toxicity of cefixime.
Keywords: Cefixime, Toxicity, Sono-electro-Fenton, Bioassay, Microorganism
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
Today, pharmaceutical compounds are universally used to treat many diseases in humans, animals, and other living organisms. These compounds have been recognized as emerging pollutants in water in the last three decades due to their diversity, consumption, and stability (1). Antibiotics are widely used in medicine and veterinary medicine as well as animal feed as growth promoters. It is estimated that 15% of all drugs used in the world are attributed to antibiotics (2). Hospitals are the main places for the production of pharmaceutical effluents due to the high consumption of antibiotics (3). Cefixime is one of these antibiotics which belongs to a class of cephalosporins and is used to treat infections such as the upper and lower respiratory tract, middle ear, paranasal sinuses, and urinary tract (4,5). Due to inadequate treatment in wastewater treatment plants, antibiotics contaminate the receiving waters, including rivers and lakes, and ultimately pose a threat to the aquatic ecosystem (3). The persistence and non-degradability of antibiotics in the environment (surface and groundwater, drinking water, municipal wastewater, and soil) lead to the bioaccumulation of these drugs and toxic effects. This is a major concern in the world and is a serious risk to human health and the environment (3,6). Considering these cases, in order to protect the environment and public health, it is necessary to remove these pollutants from aqueous solutions to lessen toxicity and control their risks.
Bioassay is a method employed to assess the toxicity of municipal and industrial wastewaters and leachates and has been recommended by the US Environmental Protection Agency to identify toxic pollutants and their effects on the environment (7,8). Toxicity assessment is used to evaluate the reaction of aquatic organisms to measure the effects of one or more toxins, wastewater, or environmental factors alone and in combination. The lower toxicity of an aqueous solution leads to better growing conditions for aquatic organisms. Due to the simplicity of work, high practical value, availability of laboratory facilities, and lower costs, this method has been used in this study (9,10).
In the last decade, advanced oxidation processes have attracted much attention to reduce the toxicity of contaminants and remove them from aqueous solutions (11). Advanced oxidation processes are processes based on the production of hydroxyl free radicals, and due to their high oxidation potential and non-selective type of pollutants, they have high efficiency in the degradation of organic matter (12-14). One of the most common methods of advanced oxidation processes is the electro-Fenton method. This method is a combination of electrocoagulation and Fenton, which uses the electrode of iron and hydrogen peroxide and leads to the formation of hydroxyl radicals, and thus the degradation of the contaminant and its removal (15). The advantages of the electro-Fenton process include high efficiency, less sludge production, simple technology, low cost, easy operation, and low toxicity of the reactants (16). On the other hand, the combined use of the electro-Fenton process and other advanced oxidation methods has led to better results in the removal of contaminants. One of these processes is the ultrasonic process, the mechanism of which is the physical degradation of contaminants based on cavities or microbubbles that result from acoustic cavitation in water. These cavities cause the production of hydroxyl and hydrogen radicals, and ultimately, the reaction of hydrogen radicals with oxygen leads to the production of water radicals (17). The combination of the electro-Fenton process with the ultrasonic process, called sono-electro-Fenton, will increase the mass transfer rate between the electrodes, clean the surface of the electrodes, reduce energy consumption, environmental compatibility, and ultimately increase the degradability of organic pollutants due to the chemical and physical reaction created (18). Due to the high toxicity caused by cefixime in aqueous solutions, the present study was performed to investigate the changes in the toxicity of cefixime by bioassay using standard strains of microorganisms during the sono-electro-Fenton process.
2. Materials and Methods
2.1. Materials Needed and Sample Preparation
Cefixime powder was prepared from Sigma Company. Stock solution (1000 mg/L) of cefixime was prepared by dissolving 1 g of cefixime in 1 L of double-distilled water. The chemical characteristics of the cefixime were shown in Table 1 (19). To adjust the pH of the solution containing cefixime, H2SO4, and NaOH (with a purity of 98%) were prepared from Merck Company (German). The standard strains of Escherichia coli and Staphylococcus aureus were prepared by the microbiology laboratory of Ardabil University of Medical Sciences, and the lactose broth for bioassay using microorganisms was provided by Pronadiza Company (Spain).
Table 1.
Characteristics of Cefixime
|
Parameter
|
Characteristics
|
| Molecular formula |
C16H15N5O7S2 |
| Molecular weight (grams per mole) |
453.452 |
| Purity |
95.6% |
| Appearance |
White powder |
| Structural formula |
|
2.2. Description of the Sono -electro-Fenton Process
This experimental study was conducted on a laboratory scale in a cylindrical reactor with a volume of 1 L, which was equipped with two iron electrodes (one as a cathode and the other as an anode). The sample volume in the reactor was 1 L, and this reactor was placed in the ultrasonic chamber with a constant frequency of 37 kHz. DC power supply (Dazheng-PS-302D model) was used to provide power. The schematic diagram of the reactor used in this study was shown in Fig. 1. One of the most common methods of advanced oxidation is the sono-electro-Fenton process. In this method, the electrodes of iron and hydrogen peroxide in the ultrasonic chamber lead to the formation of hydroxyl radicals and thus the degradation of the pollutant and its removal. Independent variables used for the sono-electro-Fenton process include pH (3-11), hydrogen peroxide (0.2-0.8 mL/L), voltage (4-16 V), initial concentration of cefixime (10-60 mg/L), and time (10-80 minutes). At the end of the reaction time, the residual concentration of cefixime at 288 nm was determined by spectrophotometer (model DR-5000, HACH, Germany), and after determining the optimal conditions, the highest cefixime removal efficiency was obtained by the sono-electro-Fenton process. Then, in the optimal conditions, the changes in toxicity of the inlet solution and the effluent treated with sono-electro-Fenton process were investigated.
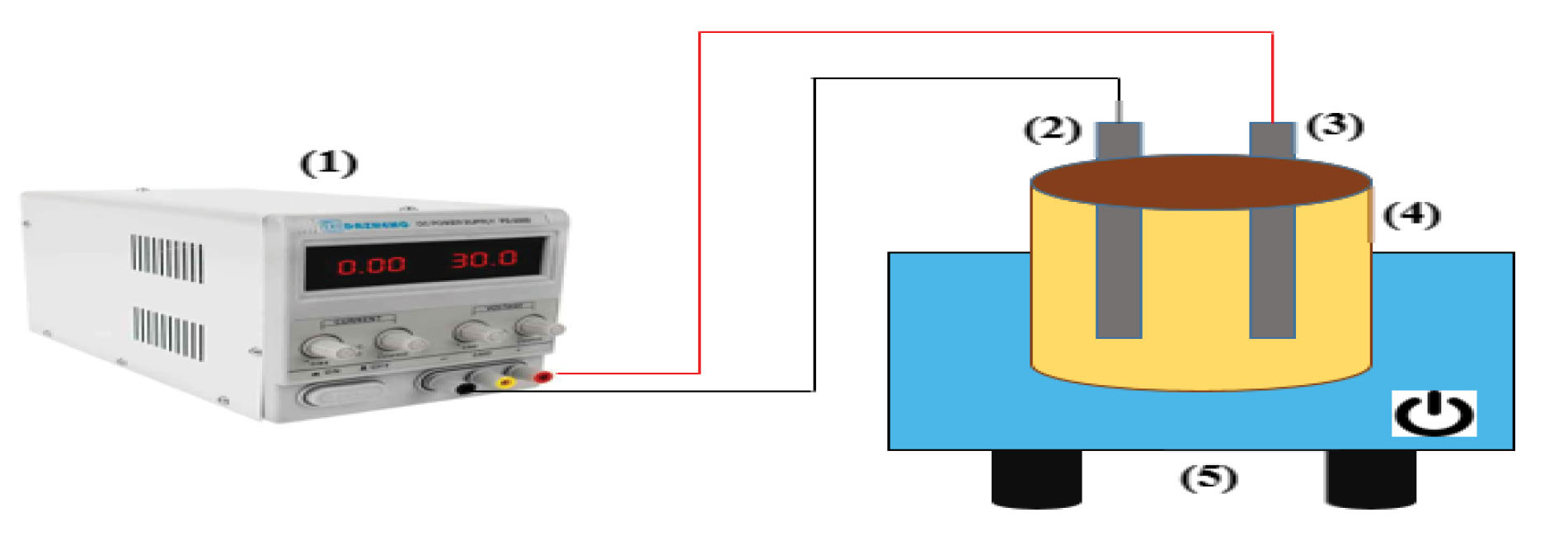
Figure 1.
Schematic Diagram of the Sono-electro-Fenton Reactor. 1) DC Power, 2) Anode, 3) Cathode, 4) Sample Tank, 5) Ultrasonic Chamber.
.
Schematic Diagram of the Sono-electro-Fenton Reactor. 1) DC Power, 2) Anode, 3) Cathode, 4) Sample Tank, 5) Ultrasonic Chamber.
2.3. Toxicity Test
In order to perform the toxicity test, the bioassay method was applied using microorganisms (20). Standard strains of E. coli (gram-negative bacteria) and S. aureus (gram-positive bacteria) were used for the bioassay test. The test procedure was as follows: First, the lactose broth was prepared according to the instructions of standard methods. Then, 40 test tubes were prepared for both bacteria (10 mL of lactose broth in each tube), and considering three repetitions, 120 test tubes containing lactose broth were prepared and were sterilized in an autoclave (temperature of 121°C, the pressure of 15 pounds per square inch, and time of 15 minutes). In the next step, E. coli was cultured on eosin methylene blue agar and S. aureus on nutrient agar medium under sterile conditions, and they were placed in an incubator at 37°C for 24 hours. For each bacterium, 5 tubes were used to test the toxicity of the inlet solution under optimal conditions (containing 10 mL of culture medium + 1 mL of the inlet solution of the reactor + 1 loop of bacteria), 5 tubes were used to test the toxicity of the outlet solution of the reactor under optimal conditions (containing 10 mL of culture medium + 1 mL of the outlet solution of the reactor + 1 loop of bacteria), and 5 tubes were selected as controls (containing 10 mL of culture medium + 1 loop of bacteria). Additionally, 5 control tubes (lactose broth only) were used to reset the spectrophotometer at 600 nm. Then, bacteria were cultured next to the flame, and one colony with a loop was removed from each tube and after inoculation and dissolution in the wall of the test tube, they were placed in an incubator at 37°C. After resetting the spectrophotometer, the absorption rate of 3 samples, i.e., the tube containing the input solution, the output solution, and the control, was read by the spectrophotometer every 2 hours. The absorbance reading was continued in 5 steps for 10 hours. Finally, the growth inhibition percentage for each bacterium in inlet and outlet solution samples was obtained by the following equation.
GI (%) = [1 - (OD600S/OD600B) × 100] (1)
In the above equation, GI represents the percentage of growth inhibition. OD600S and OD600B were the optical density of the sample and the control at a wavelength of 600 nm, respectively.
3. Results and Discussion
3.1. The Effects of Variables and Achieving Optimal Conditions
Findings related to the effect of independent variables on the son-electro-Fenton process were shown in Fig. 2. In advanced oxidation processes, especially the sono–electro-Fenton process, pH plays an important role in the removal of contaminants. In Fig. 2a, with increasing pH, the removal efficiency decreased; therefore, a higher removal percentage was observed at acidic pH. In the acidic pH range, the Fe2+ ion is more solubale, which leads to the production of hydrogen peroxide, followed by the production of hydroxyl radicals. Finally, the oxidizing power of the hydroxyl radical in acidic conditions increases, and the removal efficiency increases. In this study, pH = 3 was selected as the optimal pH.
The effects of changes in the concentration of hydrogen peroxide on the removal efficiency were represented in Fig. 2b. With increasing the amount of hydrogen peroxide from 0.2 to 0.8 mL/L, the removal efficiency increased. Based on the results, the maximum removal efficiency was observed at the concentration of 0.8 mL/L, which was selected as the optimal concentration. A normal increase in the concentration of hydrogen peroxide due to the reaction with ferrous ions leads to the production of hydroxyl radicals and an increase in oxidizing power, and the produced radicals have more access to the pollutant over time and cause more degradation of the pollutant.
The effect of the voltage on the efficiency of the sono-electro-Fenton process was investigated and it was found that increasing the voltage resulted in an increase in the removal efficiency, the results of which are shown in Fig. 2c. By increasing the voltage from 4 to 16, the removal efficiency increased from 48% to 89%, so 16 V was considered as the optimal voltage.
The effect of the initial concentration of cefixime on the removal efficiency was presented in Fig. 2d. With increasing the initial concentration of antibiotics from 10 mg/l to 60 mg/L, a diminution in removal efficiency could be detected. In the present study, the concentration of 10 mg/L was selected as the optimal concentration. By increasing the initial concentration of the contaminant, the amount of contact and exposure of the contaminant to hydroxyl radicals declines, which causes more consumption of hydroxyl radicals and decreases the removal efficiency.
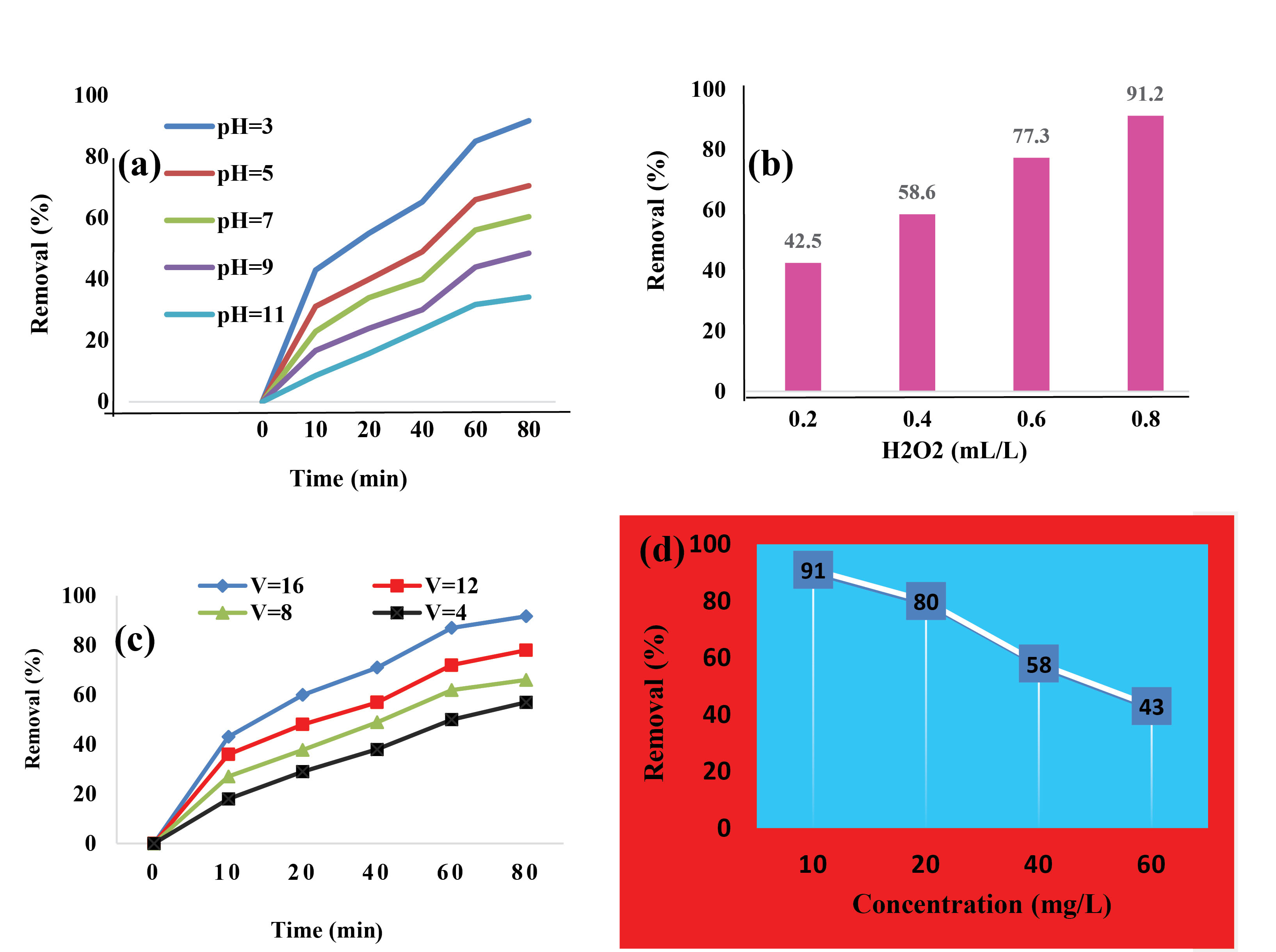
Figure 2.
The Effect of Independent Variables on the Efficiency of the Sono-electro-Fenton Process and Achieving Optimal Conditions. (a) pH, (b) H2O2 (mL/L), (c) Voltage (volt) and Electrolysis Time (min), and (d) Initial Concentration of Cefixime (mg/L).
.
The Effect of Independent Variables on the Efficiency of the Sono-electro-Fenton Process and Achieving Optimal Conditions. (a) pH, (b) H2O2 (mL/L), (c) Voltage (volt) and Electrolysis Time (min), and (d) Initial Concentration of Cefixime (mg/L).
Electrolysis time is one of the effective factors in performing advanced oxidation processes. As shown in Fig. 2c, the process efficiency increased dramatically with increasing electrolysis time. The removal efficiency improved from 35% in 10 minutes to 90% in 80 minutes. As the electrolysis time of the electrodes increases, they have more opportunity to produce ferrous ions; with increasing ferrous solubility and reaction with hydrogen peroxide, more hydroxyl radicals are produced and the removal efficiency is improved. Accordingly, in our study, 80 minutes was considered as optimal electrolysis time. Finally, under the above-mentioned optimal conditions, the removal efficiency reached 91.2% using sono-electro-Fenton process.
3.2. Toxicity Results
The results of the bioassay test conducted on the inlet solution and the effluent treated with the sono-electro-Fenton process were described in Figs. 3 and 4. In these two figures, after 10 hours, the growth rate of both bacteria in the control and effluent samples was observed to be higher; more growth indicates less toxicity. However, the growth of bacteria in the inlet solution was low, which represents the high toxicity of the inlet solution. Results of growth inhibition of the inlet solution and effluent in optimal conditions (pH = 3, H2O2 = 0.8 mL/L, voltage = 16 volts, initial concentration of antibiotic = 10 mg/L, and electrolysis time = 80 minutes) using sono-electro-Fenton process for E. coli and S. aureus bacteria are presented in Table 2. As can be seen, the toxicity (growth inhibition rate) of the inlet solution was reduced by treatment with the sono-electro-Fenton process. After 10 hours, the average growth inhibition rate for E. coli was decreased from 69.5% for the inlet solution to 11.2% for the outlet solution (83.9% reduction in toxicity), while for S. aureus, it was reduced from 25% for the inlet solution to 8% for the outlet solution (68% reduction in toxicity). In the present study, E. coli and S. aureus bacteria were used for bioassay. The reason for the use of these bacteria is their prevalence in wastewater and aquatic environments (21). In the bioassay, many organisms such as fish, algae, bacteria, and a variety of freshwater and marine organisms such as Daphnia are used. Naddafi et al applied standard strains of E. coli and S. aureus to study the toxicity of zinc oxide and titanium oxide nanoparticles using bioassay (20). In this study, the reduction in toxicity for E. coli and S. aureus in the outlet solution was 83.9% and 68%, respectively. Results of a study conducted by Rahmani et al on detoxification of sulfathiazole (30% reduction in toxicity) and sulfate methoxazole (48% reduction in toxicity) were in line with those of our study (22). Another study was conducted by Ashrafi et al on the detoxification of industrial dyes using a variety of Gram-negative and Gram-positive microorganisms; their results showed that the toxicity of effluent from the process was significantly reduced (9). In this study, E. coli was more sensitive, compared to S. aureus, which is related to the ability of gram-positive bacteria to form spores and cell wall structure (23). In the present study, the hybrid process of sono-electro-Fenton efficiently reduced the toxicity of the effluent after the decomposition of the cefixime under the optimal removal conditions. Results of studies conducted by other researchers have also shown the effective role of advanced oxidation processes in reducing the toxicity of pollutants using different species of organisms (24,25). In a study, Dirany et al evaluated the toxicity of sulfamethoxazole by bioassay using microorganisms and noted that the toxicity of the effluent treated with the electro-Fenton process was reduced by about 75%, compared to the inlet solution (26). The sono-electro-Fenton process, using a soluble fraction of iron ions and the oxidation power of hydrogen peroxide, as well as the sonic activation of ultrasonic waves, produces a hydroxyl radical that leads to the degradation of pollutants into simpler compounds such as water and carbon dioxide and beneficially reduces the biological toxicity of organic pollutants (27,28).
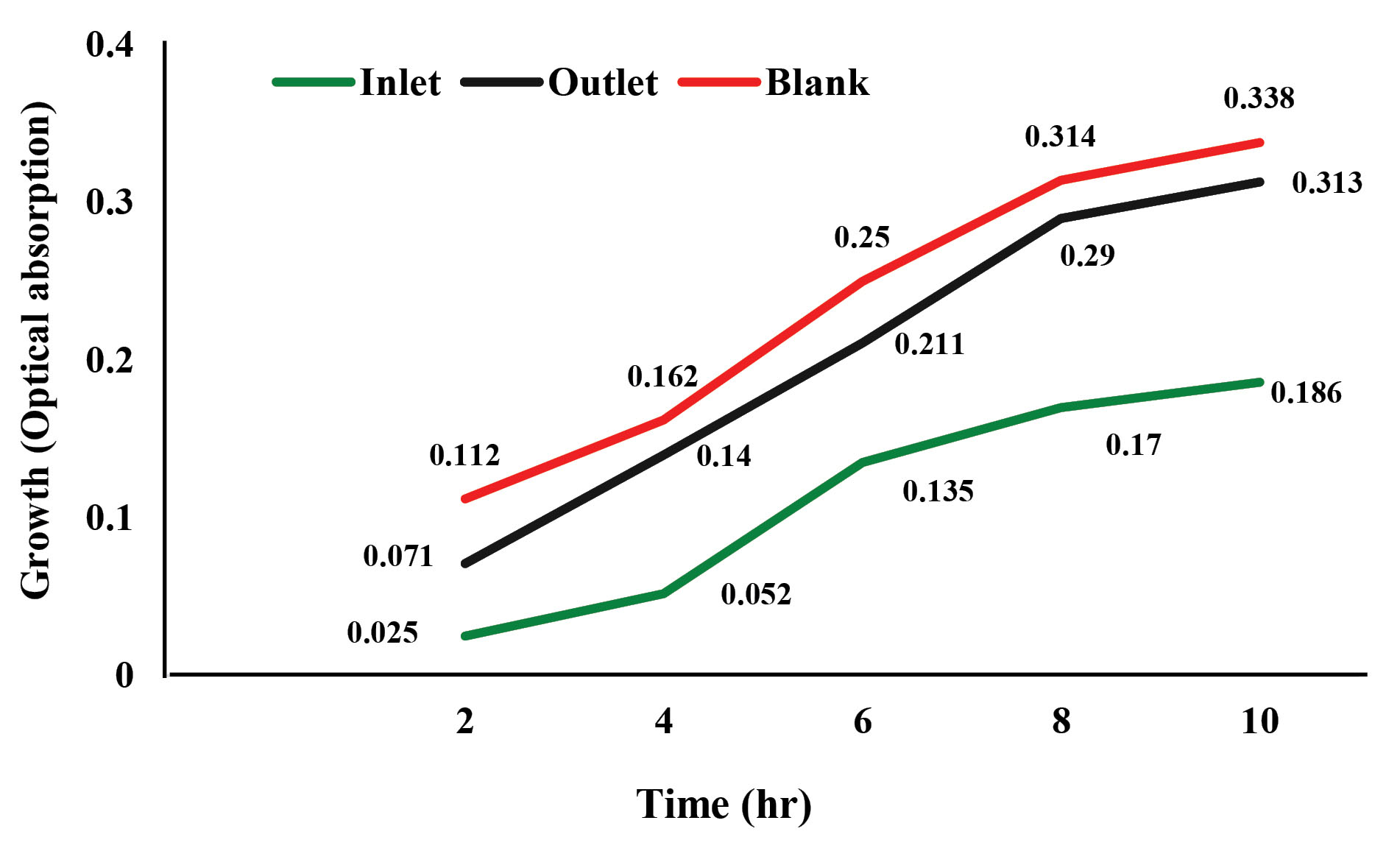
Figure 3.
Growth Trend of Escherichia Coli (Gram-negative) in Bioassay Test.
.
Growth Trend of Escherichia Coli (Gram-negative) in Bioassay Test.
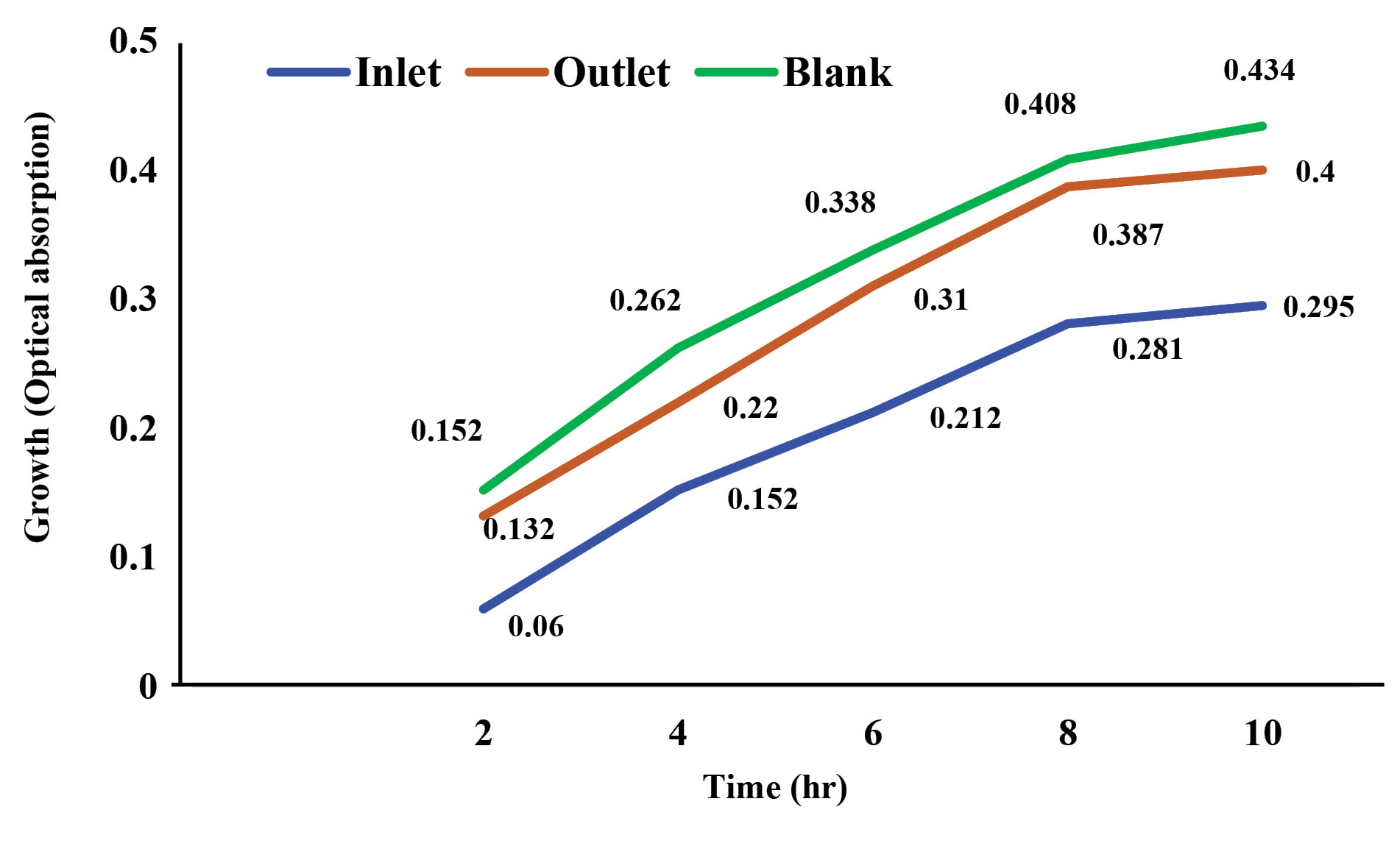
Figure 4.
Growth Trend of Staphylococcus Aureus (Gram-positive) in Bioassay Test.
.
Growth Trend of Staphylococcus Aureus (Gram-positive) in Bioassay Test.
Table 2.
Inhibition Percentage of Bacterial Growth in the Inlet and Outlet Solutions of the Reactor by Sono-electro-Fenton Process
|
Time (h)
|
Growth Inhibition Percentage of
Escherichia coli
|
Growth Inhibition Percentage of
Staphylococcus aureus
|
|
Inlet Solution
|
Outlet Solution
|
Inlet Solution
|
Outlet Solution
|
| 2 |
80.4 |
17.8 |
33.5 |
15.4 |
| 4 |
72.1 |
18.9 |
25.6 |
5.6 |
| 6 |
70.9 |
8.6 |
23.1 |
11 |
| 8 |
64 |
6.9 |
20.2 |
3.4 |
| 10 |
60.3 |
4.1 |
22.8 |
4.8 |
| Mean |
69.5 |
11.2 |
25 |
8 |
4. Conclusion
Results of our study revealed that bioassay as a useful and valuable method showed changes in the toxicity of cefixime in the inlet and outlet solutions treated with sono-electro-Fenton process using E. coli and S. aureus bacteria. In addition, the sono-electro-Fenton process, as one of the advanced oxidation methods with acceptable purification efficiency, could cause a reduction in the toxicity of cefixime in aqueous solutions.
Conflict of Interests
The authors declare that there is no conflict of interest regarding the publication of this work.
Acknowledgments
This article is a part of a research project approved by the Student Research Committee of Ardabil University of Medical Sciences with the code of IR.ARUMS.REC.1398.448. The authors would like to express their gratitude to the Vice Chancellor for Research of the University for the financial support of the project and the esteemed officials of the Laboratory of Environmental Chemistry and Microbiology of the Faculty of Health.
References
- Shokoohi R, Leili M, Dargahi A, Vaziri Y, Khamutian R. Common antibiotics in wastewater of Sina and Besat hospitals, Hamadan, Iran. Arch Hyg Sci 2017; 6(2):152-9. doi: 10.29252/ArchHygSci.6.2.152 [Crossref] [ Google Scholar]
- Alidadi H, Dolatabadi M, Mehrabpour M, Dehghan A. The efficacy of ciprofloxacin removal by Chitosan/Zeolite composite from aqueous solution: response surface methodology, kinetic and isotherm studies. Journal of Health in the Field 2017; 5(1):1-12. [ Google Scholar]
- Seifrtová M, Nováková L, Lino C, Pena A, Solich P. An overview of analytical methodologies for the determination of antibiotics in environmental waters. Anal Chim Acta 2009; 649(2):158-79. doi: 10.1016/j.aca.2009.07.031 [Crossref] [ Google Scholar]
- Ahmadi Z, Ramezani M, Sohrabi D. Effects of Cefixime on the Testis Structure and Pituitary-Gonadal Hormones in Adult Balb/C Mice. J Adv Med Biomed Res 2011; 19(74):54-62. [ Google Scholar]
- Fakhri A, Adami S. Adsorption and thermodynamic study of Cephalosporins antibiotics from aqueous solution onto MgO nanoparticles. J Taiwan Inst Chem Eng 2014; 45(3):1001-6. doi: 10.1016/j.jtice.2013.09.028 [Crossref] [ Google Scholar]
- Seid-Mohammadia A, Ghorbanianb Z, Asgaria G, Dargahic A. Photocatalytic degradation of metronidazole (MNZ) antibiotic in aqueous media using copper oxide nanoparticles activated by H. Desalination and Water Treatment 2020; 193:369-80. [ Google Scholar]
- Jin H, Yang X, Yin D, Yu H. A case study on identifying the toxicant in effluent discharged from a chemical plant. Mar Pollut Bull 1999; 39(1-12):122-5. doi: 10.1016/s0025-326x(99)00118-6 [Crossref] [ Google Scholar]
- Cairns J Jr, Buikema AL Jr, Heath AG, Parker BC. Effects of Temperature on Aquatic Organism Sensitivity to Selected Chemicals. Water Resources Research Center, Virginia Polytechnic Institute and State University; 1978.
- Ashrafi SD, Rezaei S, Forootanfar H, Mahvi AH, Faramarzi MA. The enzymatic decolorization and detoxification of synthetic dyes by the laccase from a soil-isolated ascomycete, Paraconiothyrium variabile. Int Biodeterior Biodegradation 2013; 85:173-81. doi: 10.1016/j.ibiod.2013.07.006 [Crossref] [ Google Scholar]
- Catterall K, Robertson D, Hudson S, Teasdale PR, Welsh DT, John R. A sensitive, rapid ferricyanide-mediated toxicity bioassay developed using Escherichia coli. Talanta 2010; 82(2):751-7. doi: 10.1016/j.talanta.2010.05.046 [Crossref] [ Google Scholar]
- Samarghandi MR, Dargahi A, Shabanloo A, Zolghadr Nasab H, Vaziri Y, Ansari A. Electrochemical degradation of methylene blue dye using a graphite doped PbO2 anode: optimization of operational parameters, degradation pathway and improving the biodegradability of textile wastewater. Arab J Chem 2020; 13(8):6847-64. doi: 10.1016/j.arabjc.2020.06.038 [Crossref] [ Google Scholar]
- Seidmohammadi A, Asgari G, Dargahi A, Leili M, Vaziri Y, Hayati B. A comparative study for the removal of methylene blue dye from aqueous solution by novel activated carbon based adsorbents. Prog Color Color Coat 2019; 12(3):133-44. doi: 10.30509/pccc.2019.81551 [Crossref] [ Google Scholar]
- Kestioğlu K, Yonar T, Azbar N. Feasibility of physico-chemical treatment and advanced oxidation processes (AOPs) as a means of pretreatment of olive mill effluent (OME). Process Biochem 2005; 40(7):2409-16. doi: 10.1016/j.procbio.2004.09.015 [Crossref] [ Google Scholar]
- Yazdanbakhsh AR, Manshouri M, Sheikhmohammadi A, Sardar M. Investigation the efficiency of combined coagulation and advanced oxidation by Fenton process in the removal of clarithromycin antibiotic COD. Journal of Water and Wastewater 2012; 23(2):22-9. [ Google Scholar]
- Akyol A, Can OT, Demirbas E, Kobya M. A comparative study of electrocoagulation and electro-Fenton for treatment of wastewater from liquid organic fertilizer plant. Sep Purif Technol 2013; 112:11-9. doi: 10.1016/j.seppur.2013.03.036 [Crossref] [ Google Scholar]
- Homem V, Alves A, Santos L. Amoxicillin degradation at ppb levels by Fenton’s oxidation using design of experiments. Sci Total Environ 2010; 408(24):6272-80. doi: 10.1016/j.scitotenv.2010.08.058 [Crossref] [ Google Scholar]
- Li Y, Hsieh WP, Mahmudov R, Wei X, Huang CP. Combined ultrasound and Fenton (US-Fenton) process for the treatment of ammunition wastewater. J Hazard Mater 2013; 244-245:403-11. doi: 10.1016/j.jhazmat.2012.11.022 [Crossref] [ Google Scholar]
- Ranjit PJD, Palanivelu K, Lee CS. Degradation of 2,4-dichlorophenol in aqueous solution by sono-Fenton method. Korean J Chem Eng 2008; 25(1):112-7. doi: 10.1007/s11814-008-0020-7 [Crossref] [ Google Scholar]
- Rasoulifard MH, Ghalamchi L, Azizi M, Eskandarian MR, Sehati N. Application of ultraviolet light-emitting diodes to the removal of cefixime trihydrate from aqueous solution in the presence of peroxydisulfate. J Appl Chem Res 2015; 9(3):61-72. [ Google Scholar]
- Naddafi K, Zare MR, Younesian M, Alimohammadi M, Rastkari N, Mousavi N. Bioassay for toxicity assessment of zinc oxide and titanium oxideto Escherichia coli ATCC 35218 and Staphylococcus aureus ATCC 25923 bacteria. Iran J Health Environ 2011; 4(2):171-80. [ Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater. Washington, DC: American Public Health Association (APHA); 2005.
- Rahmani K, Faramarzi MA, Mahvi AH, Gholami M, Esrafili A, Forootanfar H. Elimination and detoxification of sulfathiazole and sulfamethoxazole assisted by laccase immobilized on porous silica beads. Int Biodeterior Biodegradation 2015; 97:107-14. doi: 10.1016/j.ibiod.2014.10.018 [Crossref] [ Google Scholar]
- Sawai J, Igarashi H, Hashimoto A, Kokugan T, Shimizu M. Effect of ceramic powder slurry on spores of Bacillus subtilis. J Chem Eng Japan 1995; 28(5):556-61. [ Google Scholar]
- Le TXH, Nguyen TV, Yacouba ZA, Zoungrana L, Avril F, Petit E. Toxicity removal assessments related to degradation pathways of azo dyes: toward an optimization of electro-Fenton treatment. Chemosphere 2016; 161:308-18. doi: 10.1016/j.chemosphere.2016.06.108 [Crossref] [ Google Scholar]
- Rueda-Márquez JJ, Levchuk I, Manzano M, Sillanpää M. Toxicity reduction of industrial and municipal wastewater by advanced oxidation processes (Photo-Fenton, UVC/H2O2, Electro-Fenton and Galvanic Fenton): a review. Catalysts 2020; 10(6):612. doi: 10.3390/catal10060612 [Crossref] [ Google Scholar]
- Dirany A, Efremova Aaron S, Oturan N, Sirés I, Oturan MA, Aaron JJ. Study of the toxicity of sulfamethoxazole and its degradation products in water by a bioluminescence method during application of the electro-Fenton treatment. Anal Bioanal Chem 2011; 400(2):353-60. doi: 10.1007/s00216-010-4441-x [Crossref] [ Google Scholar]
- Babuponnusami A, Muthukumar K. Advanced oxidation of phenol: a comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem Eng J 2012; 183:1-9. doi: 10.1016/j.cej.2011.12.010 [Crossref] [ Google Scholar]
- Wang C, Shih Y. Degradation and detoxification of diazinon by sono-Fenton and sono-Fenton-like processes. Sep Purif Technol 2015; 140:6-12. doi: 10.1016/j.seppur.2014.11.005 [Crossref] [ Google Scholar]