Avicenna J Environ Health Eng. 7(1):47-52.
doi: 10.34172/ajehe.2020.07
Original Article
Electrochemical Treatment of Textile Dye Bath Wastewater Using Activated Carbon Cloth Electrodes
Zelal Isik 1  , Bahar Ozbey Unal 2, Ahmet Karagunduz 3, Bulent Keskinler 3, *, Nadir Dizge 1, *
, Bahar Ozbey Unal 2, Ahmet Karagunduz 3, Bulent Keskinler 3, *, Nadir Dizge 1, * 
Author information:
1Department of Environmental Engineering, Mersin University, Mersin, Turkey.
2Institute of Earth and Marine Sciences, Gebze Technical University, Kocaeli, Turkey.
3Department of Environmental Engineering, Gebze Technical University, Kocaeli, Turkey.
Abstract
The performance of electrooxidation (EO) treatment using activated carbon cloth (ACC) electrodes on textile dye bath wastewater was investigated. ACC electrode pairs were used as anode/cathode for EO experiments. The effect of current density (50–150 A/m2 ), operating time (0–90 minutes), and solution pH (6-11) were tested for removal of chemical oxygen demand (COD), color, and chloride, as well as the changes in conductivity. 95.5% COD and color removal efficiencies were obtained at current density (CD) of 100 A/m2 at solution pH of 10 for 90 minutes. Moreover, the chloride concentration decreased from 4254 to 35.5 mg/L and solution conductivity decreased from 160 to 131 mS/cm at the same conditions. Operating cost of the EO process was calculated to be 3.13 US$/m3 for 36 kWh/m3 energy consumption. The results indicated that the EO process with ACC electrodes achieved high pollutant removal from textile dye bath wastewater.
Keywords: Textile dye bath wastewater, Electrooxidation, Activated carbon cloth electrodes
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
A large amount of wastewater is produced daily by the textile industry that contains various types of unreacted dyes, inorganic and common organic compounds of complex pollutants, and surfactants (1). Especially, the effluent from the dyeing process contains strong color and high chemical oxygen demand (COD) levels that require satisfactory treatment for sufficient removal efficiency (2). Otherwise, if the wastewater is not treated sufficiently during the dyeing process, dye contamination and high COD levels reduce the water quality (3). Moreover, high dye and COD levels in wastewater may cause aesthetic pollution, as well as mutagenic and carcinogenic effects if the wastewater is discharged directly into the receiving environment without any treatment (4).
Several treatment technologies have been used for the treatment of textile wastewater so far. Among these methods, membrane filtration (5), photocatalytic process (6), adsorption (7), coagulation (8), advanced oxidation (9), biological processes (10), and coupled processes (11-13) are the most commonly used treatment processes. Although they have a satisfactory dye removal efficiency, some disadvantages such as an expensive investment and operating cost, formation of sludge and toxic intermediates, and membrane fouling limit the use of these technologies (4). Moreover, conventional biological processes cannot offer sufficient treatment efficiency, especially for dye removal due to the resistance of dyes to biodegradation (14). Therefore, studies on electrochemical treatment methods such as electrooxidation (EO) and electrocoagulation have increased recently due to their high dye removal efficiency in textile wastewater (2).
Electrochemical treatment processes are generally based on the indirect or mediated oxidation of the contaminants (15). The formation of hydroxyl radicals with high reactivity facilitates the degradation of the contaminants in textile wastewater (16,17). Many parameters such as solution conductivity, current density, solution pH, and electrode materials can affect electrochemical treatment processes (18). Recent studies in the literature have focused on innovative and environmentally friendly electrodes in which activated carbon cloth (ACC) electrodes have become prominent due to their high mechanical strength, easy handling, regeneration properties, and direct usage potential (19). While there are quite a lot of studies on the adsorption and electrosorption using ACC, studies on the use of electrochemical processes have just begun (20,21). Gineys et al (21) investigated the effect of polarization on the nanotexture of the ACC which can be used as an electrode in the electrochemical processes. It was observed that the oxidation of the pristine carbon material was enhanced by anodic polarization while the surface chemistry and the properties of the nanotexture were not affected too much by cathodic polarization. Moreover, Wang et al (2) studied the removal of COD from real dye wastewater by the electro-Fenton process in which hydrogen peroxide was generated by polyacrylonitrile-based activated carbon fiber cloth cathode. They observed that COD removal efficiency was over 70% for 240 minutes of treatment. Additionally, they reported that the higher current densities deteriorated the COD removal efficiency due to the generation of side reactions.
Chloride rich cotton textile dye bath dump wastewater was treated using electrocoagulation process (22). Four electrode combinations (stainless steel-stainless steel, iron-iron, aluminium-aluminium, and iron-aluminium) were tested on the removal of color and COD concentration. Iron electrodes enhanced efficient treatment and 98.8% color removal efficiency was observed at 12 V and 15 minutes treatment time with a sludge generation rate of 22.7 g/L. Moreover, 80.3% COD removal efficiency was obtained at 5.8 V and 5.7 minutes treatment time with a sludge generation rate of 5.7 g/L (22). In another study, textile dye wastewater was treated with electrochemical oxidation in a batch reactor. A maximum COD removal efficiency of 97.17% was obtained at a reactor volume of 300 mL, electrolysis time of 6 hours, and current density of 4.0 A/dm2(23).
This study focused on investigating the performance and feasibility of treatment of textile dye bath wastewater by EO process using ACC electrodes. The effect of current density (50–150 A/m2), operating time (0–90 minutes), and pH of the initial wastewater (6-11) were systematically investigated for the removal of COD, color, and chloride, as well as the changes in conductivity. Operating cost and energy consumption of the EO process were also examined.
2. Materials and Methods
2.1. Characterization of Textile Dye Bath Wastewater
Textile dye bath wastewater was kindly provided by a large-scale textile industry (Seckin Textile, Gaziantep, Turkey). The produced dye bath wastewater quantity was about 50 m3/d. Samples were collected weekly from January to February 2020. Wastewater was used as received and it was not diluted during the experiments. The characterization of textile dye bath wastewater is given in Table 1.
Table 1.
Characterization of Textile Dye Bath Wastewater
|
Sampling Date
|
pH
|
Conductivity
(mS/cm)
|
Color
(Pt-Co)
|
Chloride
(mg/L)
|
COD
(mg/L)
|
| 05.01.2020 |
11.1 |
160 |
3320 |
4254 |
5312 |
| 15.01.2020 |
11.0 |
155 |
3300 |
4500 |
5300 |
| 30.01.2020 |
11.3 |
162 |
3330 |
4375 |
5305 |
| 05.02.2020 |
11.5 |
158 |
3325 |
4450 |
5325 |
2.2. Experimental Setup
The schematic illustration of the batch reactor setup is shown in Fig. 1. It was composed of a borosilicate glass reactor, electrodes, a digital DC power system, connecting wires, a magnetic stirrer, and a water bath. EO experiments were performed in a 500 mL reactor with a working volume of 250 mL. The reactor was placed in a temperature-controlled water bath to supply a constant reaction temperature (25±1°C). A magnetic stirrer (Wisd -Wisestir MSH-20A) and a Teflon-covered magnetic stirring bar were used to mix the wastewater at 300 rpm. ACC was used as anode/cathode electrode pairs. The ACC electrodes were kindly provided by Norm Technologies, Turkey. The dimensions of anode/cathode electrode pairs were arranged as 5 cm width × 8 cm high × 1 mm thickness with the total effective area of 40 cm2and distance of 2 cm between the electrodes. DC power source (AATech ADC-3303D, the maximum voltage of 30 V) was used to set anode and cathode connected to the positive and negative outlets.

Figure 1.
Experimental Set-up for Electrooxidation (1. Magnetic Stirrer, 2. Magnetic Bar, 3. Glass Reactor, 4. Anode ACC Electrode, 5. Cathode ACC Electrode, 6. DC Power Source).
.
Experimental Set-up for Electrooxidation (1. Magnetic Stirrer, 2. Magnetic Bar, 3. Glass Reactor, 4. Anode ACC Electrode, 5. Cathode ACC Electrode, 6. DC Power Source).
2.3. Analysis
Four different current densities (50, 75, 100, and 150 A/m2), five different EO times (15, 30, 45, 60, and 90 minutes), and four different wastewater pH values (6, 8, 10, and 11) were tested for EO treatment of textile dye bath wastewater. At appropriate time intervals, samples were taken from the reactor, centrifuged at 6000 rpm for 5 minutes, and analyzed to measure pH, conductivity, COD, color, and chloride. The conductivity and pH were measured using a pH/Cond 340i Handheld Multimeters, WTW. COD was measured according to Standard Method No. 5220C (24). The color was measured by Platinum–Cobalt (Pt–Co) method in accordance with Standard Method No. 2120 (24). The chloride concentration was measured by Argentometric method in accordance with Standard Method No. 4500B (24). All EO experiments were performed in duplicates.
Removal efficiencies of color and COD were calculated using (Equation 1):
(1)
where Ci was the initial concentration and Cf was the concentration after defined reaction time t (min).
3. Results and Discussion
EO was used as an electrochemical treatment for textile dye bath wastewater treatment using ACC electrode pairs as the anode/cathode electrodes. The effect of current density (CD) and solution pH were investigated on COD and color removal efficiencies as well as variations of the solution conductivity and chloride concentration. Energy and cost analyses were also presented. The detailed explanation is given as follows.
3.1. The Effect of Current Density on Removal Efficiencies
The electrochemical reactor was operated under different current density (50, 75, 100, 150 A/m2) conditions. COD and color removal efficiencies as well as variations of the chloride concentration and solution conductivity were affected when the current density was increased. The COD and color removal efficiencies changed in the range of 73.6%–79.1% (Fig. 2A) and 21.7%–80.6% (Fig. 2B) for 50–150 A/m2 current density, respectively. Chloride concentration and solution conductivity changed in the range of 70.4–212.7 mg/L (Fig. 2C) and 128–144 mS/cm (Fig. 2D) for 50–150 A/m2 current density, respectively. pH increased from 11.4 to 11.8 when the applied current density was increased from 50 to 150 A/m2(Fig. 2E). The results showed that the COD and color removal efficiencies decreased when the applied current density reached up to 150 A/m2, denoting that the electrogeneration rate of hypochlorite decreased. It is well known that chlorides (Cl-) are the most widespread species for the mediated oxidation and textile dye bath wastewater includes chlorides which can be easily converted into chlorine (Cl2) and hypochlorite according to the reaction of (Eq. 2) at the anode (25). Additionally, the main reaction at the cathode is given in (Equation 3) (26).
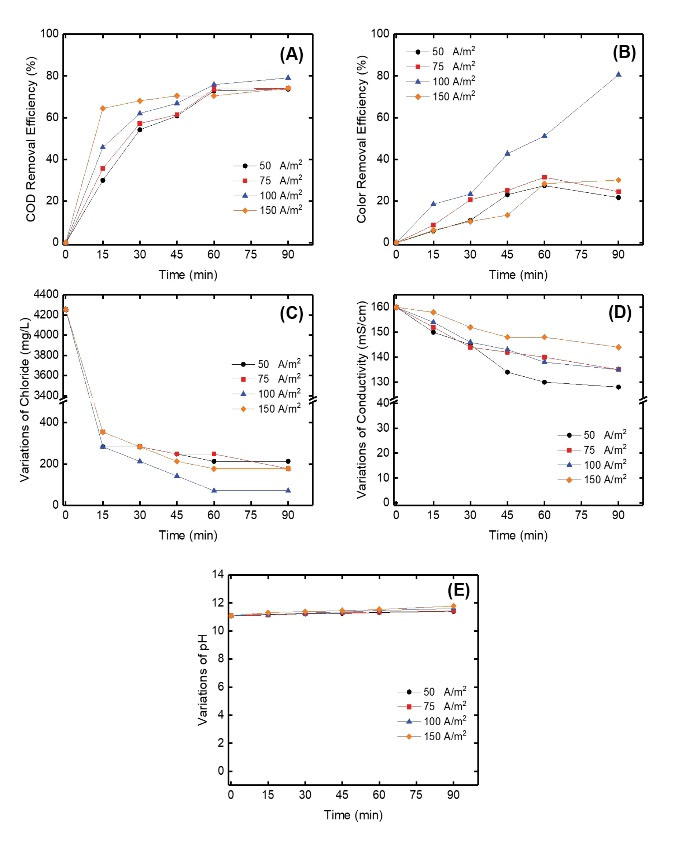
Figure 2.
The Effect of Current Density on (A) COD Removal Efficiency, (B) Color Removal Efficiency, (C) Variations of Chloride Concentration, (D) Variations of Conductivity, and (E) Variations of pH During the Reaction.
.
The Effect of Current Density on (A) COD Removal Efficiency, (B) Color Removal Efficiency, (C) Variations of Chloride Concentration, (D) Variations of Conductivity, and (E) Variations of pH During the Reaction.
The discharged Cl2 gas is hydrolyzed and ionized as the reactions given in (Eq. 4) and (Eq. 5);
In addition, the side reactions occurred in anode via the formed O2 which caused the formation of chlorate (ClO3-) according to (Eq. 6) and (Eq. 7);
(7)
At higher pH (11.1 in our case), OCl- species controls the oxidation reaction and enhances the color and COD removal efficiency (22,27). Although hypochlorite (ClO−) is the key product, some intermediate reactions such as chlorine (Cl2) and hypochlorous acid (HOCl) are also formed (28). These reactive species tend to react quickly with many organic compounds and promote their mineralization (29).
At the end of the reaction, we did not observe any sludge in the reaction medium and any electrode solubility in this study.
3.2. The Effect of Solution pH on Removal Efficiencies
The electrochemical reactor was operated under different pH (6, 8, 10, and 11) conditions. COD and color removal efficiencies as well as variations of the chloride concentration and solution conductivity were affected by changing the solution pH. The COD and color removal efficiencies increased in the range of 79.1%–95.5% (Fig. 3A) and 80.6%–95.5% (Fig. 3B) for solution pH of 6–11, respectively. Chloride concentration and solution conductivity changed in the range of 106.3–35.4 mg/L (Fig. 3C) and 144–131 mS/cm (Fig. 3D) for solution pH of 6–11, respectively. However, pH increased from 6.3 to 11.6 when the solution pH was increased from 6 to 11(Fig. 3E).
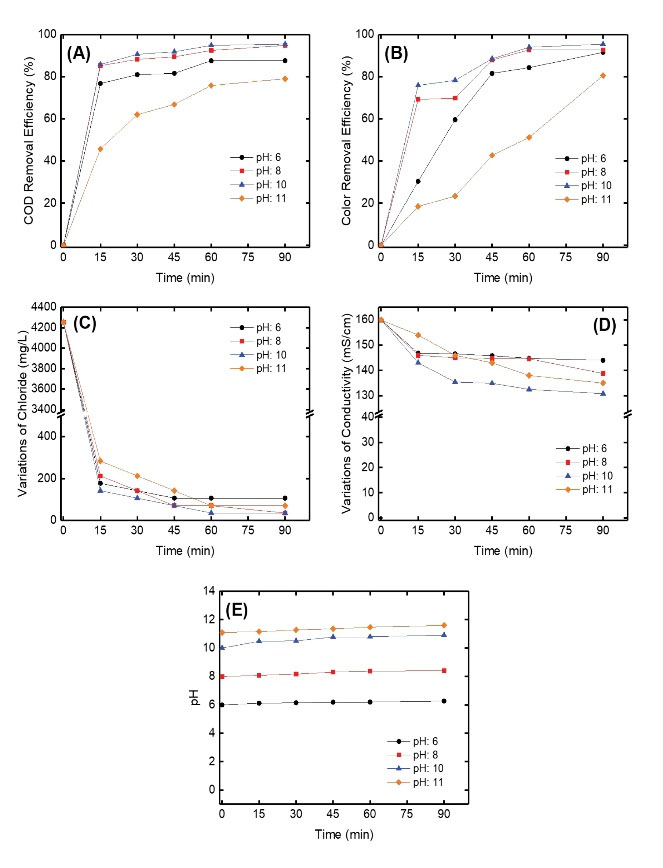
Figure 3.
The Effect of Solution pH on (A) COD Removal Efficiency, (B) Color Removal Efficiency, (C) Variations of Chloride Concentration, (D) Variations of Conductivity, and (E) Variations of pH During the Reaction.
.
The Effect of Solution pH on (A) COD Removal Efficiency, (B) Color Removal Efficiency, (C) Variations of Chloride Concentration, (D) Variations of Conductivity, and (E) Variations of pH During the Reaction.
HOCl supplies 84% of the ionic species at pH 6.8, but only 0.34% at pH 10. The primary species is the ClO- ion at pH 10 (30). In our study, we obtained lower COD and color efficiencies at low pH value (pH=6) compared to higher pH values except for pH=11. It can be explained that ionization of HOCl species decreases at lower pH values, leading to a decrease in the oxidation efficiency. The reaction of Cl2 in acidic and alkaline medium is given by (Equation 8) and (Equation 9).
(acidic medium) (8)
(alkaline medium) (9)
The reaction of
is incomplete at pH 5.0-8.5 and both species are present to some degree (31). When the pH goes down from 8 to 6, it is obvious that H+ ions become readily available again and the ClO-ions return to HOCl, which is the active or killing form of chlorine. Therefore, this form of chlorine decreased the available oxidant and negatively affected COD and color removal efficiencies.
The presence of chloride ions in the wastewater causes an increase in contaminant removal efficiency because of the formation of active chlorine. In an alkaline medium, the hydrolyzed Cl2 gases produce the hypochlorite ions that can oxidize the organics in the anode and/or in the bulk of the solution (Eq. 10) (32,33).
(10)
3.3. Energy Consumption and Cost Analysis
The operating cost is one of the most important parameters for wastewater treatment plants.
Generally, the cheapest and most effective processes are preferred for wastewater treatment plants (34). The formula of energy consumption is given by (Eq. 11):
where EEO (kWh/m3) is electrical energy consumption, V is voltage (V), I is current (A), t is time (h), and VR is wastewater volume (m3) (1 kWh = 0.074 US$ according to Turkish Electricity Distribution Company for the date 05.06.2020). Energy consumptions for different current densities and solution pH values are given in Figs. 4A and 4C, respectively. Cost analyses for different current densities and solution pH values are shown in Figs. 4B and 4D, respectively. The optimum energy consumption and energy cost were calculated to be 36 kWh/m3(Fig. 4C) and 2.66 US$/m3(Fig. 4D), respectively, for current density of 100 A/m2and solution pH of 10.
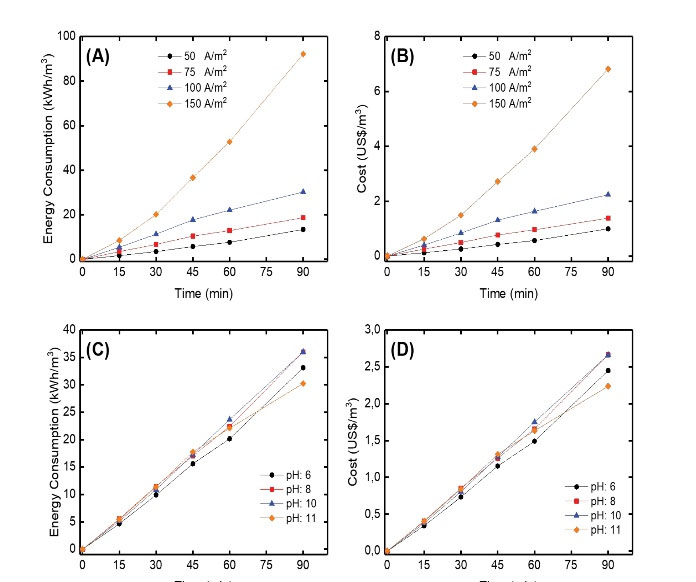
Figure 4.
(A)Energy Consumption and (B) Cost Analysis for Different Current Densities. (C) Energy Consumption and (D) Cost Analysis for Different Solution pH Values.
.
(A)Energy Consumption and (B) Cost Analysis for Different Current Densities. (C) Energy Consumption and (D) Cost Analysis for Different Solution pH Values.
The photographs of treated textile dye bath wastewater are shown in Fig. 5. Color density decreased dramatically after 90 minutes of electrooxidation.

Figure 5.
The Photographs of Treated Textile Dye Bath Wastewater after Different Electrolysis Times.
.
The Photographs of Treated Textile Dye Bath Wastewater after Different Electrolysis Times.
Table 2 shows a performance comparison between the ACC electrode used in this study and the previously reported other electrodes for textile dye wastewater treatment using EO process. As shown, the use of ACC electrodes has considerable advantages over the reported methods considering color and COD removal efficiencies. Additionally, using ACC electrodes has other advantages such as simplicity, cost-effectiveness, no sludge production, and environmentally-friendliness.
Table 2.
The Performance Comparison of the Electrodes in the Electrooxidation Process for Textile Dye Bath Wastewater Treatment
|
Electrode
|
Experimental Conditions
|
Removal Efficiency (%)
|
Energy Consumption
|
Ref.
|
Anode: Ti/Pt
Cathode: Stainless steel 304 |
EO time: 18 min
CD: 0.89 A/cm2 |
COD: 86%
Color: 100% |
21 kWh/kg COD |
(35) |
Anode: Pt
Cathode: Pt |
EO time: 60 min
Voltage: 12 V |
COD: 35.28% |
0.054 kWh/m3 |
(36) |
Anode: Pt cage
Cathode: Pt foil |
EO time: 90 min
Voltage: 40 V |
COD: 60%
Color: 100% |
̶ |
(37) |
Anode: ACC
Cathode: ACC |
EO time: 90 min
Voltage: 100 A/m2 |
COD: 95.5%
Color: 95.5% |
36 kWh/m3 |
This study |
4. Conclusion
Textile wastewater may contain some toxic dyes and must be treated efficiently before being released into the receiving environment. Additionally, certain dyes are assumed to have carcinogenic effects on humans and living organisms. In the present study, optimization of operational parameters in the electrochemical oxidation process for treatment of textile dye bath wastewater using ACC electrodes was performed successfully. The effects of operational parameters such as current density, solution pH, and electrolysis time were investigated on COD and color removal efficiencies, as well as variations of chloride concentration and solution conductivity. High COD and color removal efficiencies (95.5%) were achieved at the current density of 100 A/m2and solution pH of 10 for 90-minute electrolysis time. Energy consumptions and energy cost were calculated to be 36 kWh/m3and 2.66 US$/m3, respectively. In the optimized experimental conditions, the treatment of textile dye bath wastewater by electrochemical oxidation method using ACC as an anode is suitable for the removal of color and COD.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
Ethical Statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee.
Acknowledgements
The authors would like to thank all those who contributed to this research.
References
- GilPavas E, Dobrosz-Gómez I, Gómez-García M. Coagulation-flocculation sequential with Fenton or Photo-Fenton processes as an alternative for the industrial textile wastewater treatment. J Environ Manage 2017; 191:189-97. doi: 10.1016/j.jenvman.2017.01.015 [Crossref] [ Google Scholar]
- Wang CT, Chou WL, Chung MH, Kuo YM. COD removal from real dyeing wastewater by electro-Fenton technology using an activated carbon fiber cathode. Desalination 2010; 253(1):129-34. doi: 10.1016/j.desal.2009.11.020 [Crossref] [ Google Scholar]
- Yaseen DA, Scholz M. Textile dye wastewater characteristics and constituents of synthetic effluents: a critical review. Int J Environ Sci Technol 2019; 16(2):1193-226. doi: 10.1007/s13762-018-2130-z [Crossref] [ Google Scholar]
- Aravind P, Subramanyan V, Ferro S, Gopalakrishnan R. Eco-friendly and facile integrated biological-cum-photo assisted electrooxidation process for degradation of textile wastewater. Water Res 2016; 93:230-41. doi: 10.1016/j.watres.2016.02.041 [Crossref] [ Google Scholar]
- Rashidi HR, Sulaiman NMN, Hashim NA, Hassan CRC, Ramli MR. Synthetic reactive dye wastewater treatment by using nano-membrane filtration. Desalin Water Treat 2015; 55(1):86-95. doi: 10.1080/19443994.2014.912964 [Crossref] [ Google Scholar]
- Hussein FH, Abass TA. Photocatalytic treatment of textile industrial wastewater. Int J Chem Sci 2010; 8(3):1353-64. [ Google Scholar]
- Bharathi KS, Ramesh ST. Removal of dyes using agricultural waste as low-cost adsorbents: a review. Appl Water Sci 2013; 3(4):773-90. doi: 10.1007/s13201-013-0117-y [Crossref] [ Google Scholar]
- Szyguła A, Guibal E, Ariño Palacín M, Ruiz M, Sastre AM. Removal of an anionic dye (Acid Blue 92) by coagulation-flocculation using chitosan. J Environ Manage 2009; 90(10):2979-86. doi: 10.1016/j.jenvman.2009.04.002 [Crossref] [ Google Scholar]
- Nawaz MS, Ahsan M. Comparison of physico-chemical, advanced oxidation and biological techniques for the textile wastewater treatment. Alex Eng J 2014; 53(3):717-22. doi: 10.1016/j.aej.2014.06.007 [Crossref] [ Google Scholar]
- Bhatia D, Sharma NR, Singh J, Kanwar RS. Biological methods for textile dye removal from wastewater: a review. Crit Rev Environ Sci Technol 2017; 47(19):1836-76. doi: 10.1080/10643389.2017.1393263 [Crossref] [ Google Scholar]
- Buscio V, García-Jiménez M, Vilaseca M, López-Grimau V, Crespi M, Gutiérrez-Bouzán C. Reuse of textile dyeing effluents treated with coupled nanofiltration and electrochemical processes. Materials (Basel) 2016;9(6). 10.3390/ma9060490.
- Han G, Liang C-Z, Chung T-S, Weber M, Staudt C, Maletzko C. Combination of forward osmosis (FO) process with coagulation/flocculation (CF) for potential treatment of textile wastewater. Water Res 2016; 91:361-70. doi: 10.1016/j.watres.2016.01.031 [Crossref] [ Google Scholar]
- Paździor K, Bilińska L, Ledakowicz S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem Eng J 2019; 376:120597. doi: 10.1016/j.cej.2018.12.057 [Crossref] [ Google Scholar]
- Liang J, Ning XA, Sun J, Song J, Hong Y, Cai H. An integrated permanganate and ozone process for the treatment of textile dyeing wastewater: Efficiency and mechanism. J Clean Prod 2018; 204:12-9. doi: 10.1016/j.jclepro.2018.08.112 [Crossref] [ Google Scholar]
- Radjenovic J, Sedlak DL. Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water. Environ Sci Technol 2015; 49(19):11292-302. doi: 10.1021/acs.est.5b02414 [Crossref] [ Google Scholar]
- Raju GB, Karuppiah MT, Latha SS, Parvathy S, Prabhakar S. Treatment of wastewater from synthetic textile industry by electrocoagulation–electrooxidation. Chem Eng J 2008; 144(1):51-8. doi: 10.1016/j.cej.2008.01.008 [Crossref] [ Google Scholar]
- Orts F, del Río AI, Molina J, Bonastre J, Cases F. Study of the reuse of industrial wastewater after electrochemical treatment of textile effluents without external addition of chloride. Int J Electrochem Sci 2019; 14(2):1733-50. doi: 10.20964/2019.02.27 [Crossref] [ Google Scholar]
- Orts F, Bonastre J, Fernández J, Cases F. Effect of chloride on the one step electrochemical treatment of an industrial textile wastewater with tin dioxide anodes The case of trichromy procion HEXL. Chemosphere 2020; 245:125396. doi: 10.1016/j.chemosphere.2019.125396 [Crossref] [ Google Scholar]
- Cukierman AL. Development and environmental applications of activated carbon cloths. ISRN Chem Eng 2013; 2013:261523. doi: 10.1155/2013/261523 [Crossref] [ Google Scholar]
- Le Cloirec P. Adsorption onto activated carbon fiber cloth and electrothermal desorption of volatile organic compound (VOCs): a specific review. Chin J Chem Eng 2012; 20(3):461-8. doi: 10.1016/s1004-9541(11)60207-3 [Crossref] [ Google Scholar]
- Gineys M, Benoit R, Cohaut N, Béguin F, Delpeux-Ouldriane S. Behavior of activated carbon cloths used as electrode in electrochemical processes. Chem Eng J 2017; 310:1-12. doi: 10.1016/j.cej.2016.10.018 [Crossref] [ Google Scholar]
- Singh H, Bhatti MS, Reddy AS. Decolourization of textile dyebath chloride rich wastewater by electrolytic processes. Int J Electrochem Sci 2017; 12(5):3662-74. doi: 10.20964/2017.05.63 [Crossref] [ Google Scholar]
- Sendhil J, Muniswaran P, Basha CA. Real textile dye wastewater treatment by electrochemical oxidation: application of response surface methodology (Rsm). Int J ChemTech Res 2014; 7(6):2681-90. [ Google Scholar]
- Rice EW, Baird RB, Eaton AD. Standard Methods for the Examination of Water and Wastewater. 23rd ed. American Public Health Association, American Water Works Association, Water Environment Federation; 2017.
- Sirés I, Brillas E, Oturan MA, Rodrigo MA, Panizza M. Electrochemical advanced oxidation processes: today and tomorrow A review. Environ Sci Pollut Res Int 2014; 21(14):8336-67. doi: 10.1007/s11356-014-2783-1 [Crossref] [ Google Scholar]
- Basha CA, Sendhil J, Selvakumar KV, Muniswaran PKA, Lee CW. Electrochemical degradation of textile dyeing industry effluent in batch and flow reactor systems. Desalination 2012; 285:188-97. doi: 10.1016/j.desal.2011.09.054 [Crossref] [ Google Scholar]
- Deborde M, von Gunten U. Reactions of chlorine with inorganic and organic compounds during water treatment-kinetics and mechanisms: a critical review. Water Res 2008; 42(1-2):13-51. doi: 10.1016/j.watres.2007.07.025 [Crossref] [ Google Scholar]
- Jia Z, Margerum DW, Francisco JS. General-acid-catalyzed reactions of hypochlorous acid and acetyl hypochlorite with chlorite ion. Inorg Chem 2000; 39(12):2614-20. doi: 10.1021/ic991486r [Crossref] [ Google Scholar]
- Kishimoto N, Nishimura H. Effect of pH and molar ratio of pollutant to oxidant on a photochemical advanced oxidation process using hypochlorite. Environ Technol 2015; 36(19):2436-42. doi: 10.1080/09593330.2015.1034187 [Crossref] [ Google Scholar]
- Niedz RP, Bausher MG. Control of in vitro contamination of explants from greenhouse-and field-grown trees. In Vitro Cell Dev Biol Plant 2002; 38(5):468-71. doi: 10.1079/ivp2002316 [Crossref] [ Google Scholar]
- Gray NF. Chapter Thirty-One - Free and combined chlorine. In: Percival SL, Yates MV, Williams DW, Chalmers RM, Gray NF, eds. Microbiology of Waterborne Diseases. 2nd ed. London: Academic Press; 2014. p. 571-90. 10.1016/B978-0-12-415846-7.00031-7.
- Scialdone O, Galia A, Sabatino S. Abatement of Acid Orange 7 in macro and micro reactors Effect of the electrocatalytic route. Applied Catalysis B: Environmental 2014; 148-149:473-83. doi: 10.1016/j.apcatb.2013.11.005 [Crossref] [ Google Scholar]
- Ghanbari F, Moradi M. Electrooxidation processes for dye degradation and colored wastewater treatment. In: Gautam RK, Chattopadhyaya MC, eds. Advanced Nanomaterials for Wastewater Remediation. CRC Press; 2016. p. 61-108. 10.1201/9781315368108.
- Dizge N, Akarsu C, Ozay Y, Gulsen HE, Adiguzel SK, Mazmanci MA. Sono-assisted electrocoagulation and cross-flow membrane processes for brewery wastewater treatment J Water Process. Eng 2018; 21:52-60. doi: 10.1016/j.jwpe.2017.11.016 [Crossref] [ Google Scholar]
- Vlyssides AG, Loizidou M, Karlis PK, Zorpas AA, Papaioannou D. Electrochemical oxidation of a textile dye wastewater using a Pt/Ti electrode. J Hazard Mater 1999; 70(1-2):41-52. doi: 10.1016/s0304-3894(99)00130-2 [Crossref] [ Google Scholar]
- Jović M, Stanković D, Manojlović D, Andelković I, Milić A, Dojćinović B. Study of the electrochemical oxidation of reactive textile dyes using platinum electrode. Int J Electrochem Sci 2013; 8(1):168-83. [ Google Scholar]
- Doğan D, Türkdemir H. Electrochemical oxidation of textile dye indigo. J Chem Technol Biotechnol 2005; 80(8):916-23. doi: 10.1002/jctb.1262 [Crossref] [ Google Scholar]