Avicenna J Environ Health Eng. 7(2):72-77.
doi: 10.34172/ajehe.2020.11
Original Article
Sorption of Acid Orange 3 From Aqueous Solution Using Eggshell
Mohammad Hossien Saghi 1  , Marziyeh Mohamadi 1, Sakineh Rouki 1, Hamze Salehzadeh 2, Shahram Sadeghi 2, Mohammad Hasan Kowsari 1, *
, Marziyeh Mohamadi 1, Sakineh Rouki 1, Hamze Salehzadeh 2, Shahram Sadeghi 2, Mohammad Hasan Kowsari 1, * 
Author information:
1Department of Environmental Health Engineering, School of Public Health Sabzevar University of Medical Sciences, Sabzevar, Iran.
2Department of Environmental Health Engineering, Environmental Health Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran.
Abstract
Dyes are used extensively in textile industries. The wastewater of these industries contains high amounts of pollutants which can be toxic, carcinogenic, and mutagenic and needs to be treated before being discharged into the environment. The aim of this study was to evaluate the application of eggshell as a sorbent for the removal of Acid Orange 3 from synthetic wastewater. This study is applied experimental research which was performed on a laboratory scale. Eggshell as a sorbent was prepared at laboratory temperature and pulverized by standard ASTM sieves in two sizes (50 and 140). The concentration of dye in the synthetic wastewater was 25 mg/L. In this study, increasing adsorbent dose from 2 to 5 g/100 mL led to an increase in the adsorption efficiency from 36.6% to 55.36% and by decreasing the size of eggshell particles from 50 to 140 mesh, the adsorption efficiency increased. The maximum adsorption took place in the first 90 minutes of the reaction. By increasing pH from 5 to 9, the process efficiency increased from 78 to 82%; however, at pH higher than 9, the adsorption efficiency decreased. Additionally, the adsorption characteristics of this pollutant on eggshell fitted Freundlich isotherm (R2 >0.989). Due to the characteristics of textile wastewater such as alkaline pH, eggshells can be used as a natural adsorbent in textile wastewater treatment.
Keywords: Textile wastewater, Acid orange 3, Adsorbent, Eggshell
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
The dyeing, printing, and washing steps lead to the high consumption of water in the textile industry (1). Generally, depending on the process, water consumption in these industries is between 25 and 250 cubic meters per ton of product (2). Because of their complex structure, colors are often stable and resistant to degradation, and they are often toxic, carcinogenic, and mutagenic. Color is one of the most important pollutants in the textile industry (3-5). For this reason, sewage is one of the most important threats to public health and the environment around the world (6). The presence of color pollutants in amounts of less than 1 mg/L is also apparently visible and important (7). Therefore, these types of pollutants should be eliminated before being discharged from the textile industry into the environment (8). Azo dyes are a class of colored organic compounds that have largely been used in industry for many applications such as textiles, papers, leathers, additives, and analytical chemistry. One of the problems concerning industrial effluent, particularly those of textile industries, is the existence of dye. Therefore, it is mandatory to treat these effluents before being discharged into the environment (9). Similar to most of the azo dyes, Acid Orange 3 tends to be disposed in industrial wastewater, thereby imposing health threats to humans. It is highly toxic, and its ingestion can cause eye, skin, mucous membrane, and upper respiratory tract irritations, severe headaches, dizziness, nausea, and loss of bone marrow leading to anemia. The discharge of wastewater contaminated with AO azo dyes is strictly regulated in many countries for aesthetic reasons and because of breakdown products which are toxic and mutagenic (10).
Because of the resistance of dyes to biodegradation, physical and chemical methods such as coagulation, flocculation, adsorption, and oxidation are often used (11,12). The use of surface adsorbents to remove colors from the textile industry is one of the most effective methods for removing wastewater from the textile industry. In recent years, many adsorbents have been used, such as activated carbon, date fibers, sawdust, barley bran, rice husk, and chitosan, to remove colors (13-16). Natural adsorbents can be used in the process of removing colors as cheap adsorbent. Eggshell is one of these materials which can be used as an adsorbent because of its cavity structure. The eggshell structure has 4% organic compounds, 94% calcium carbonate, and 2% calcium phosphate and magnesium carbonate (17-19). The eggshell has been used as adsorbent to remove copper, lead, nickel, and cadmium from aqueous solutions (20). In a study by Ghaneian et al on the removal of red reactive dye 123 from synthetic textile wastewater using eggshells, it was revealed that this compound can remove red reactive dye by 80.7% at an adsorbent dose of 5 gr/100 mL (21). Moreover, Zheng et al conducted a study on hydroxyapatite carbonate which was synthesized from the eggshell as a metal adsorbent for the removal of cadmium and copper (II). The results showed that this adsorbent had a removal efficiency of 94 and 93.17% for cadmium and copper, respectively (22). Acid Orange 3 is a mono-azo compound, which is used extensively in the domestic textile industry. Considering the high volume of colored sewage production in the country and its adverse effects, it is necessary to develop scientific solutions to sewage treatment. Based on the environmental and economic importance of the issue, this study aimed to remove Acid Orange 3 from synthetic textile wastewater using eggshell (23).
2. Materials and Methods
This study was experimental laboratory research performed at the Chemistry Laboratory of the Sabzevar University of Medical Sciences. After preparing eggshells to remove primary impurities, the eggshells were washed with distilled water (twice distillation) and dried at laboratory temperature (25°C). An electric mill was used to grind eggshells for 10 seconds, and the ground shells were then graded by standard ASTM sieve. For this purpose, the shells were characterized by sieve mesh sizes 50, 100, 140, and 400 and the particles passing through the sieve 50 and remaining on the sieve 100 were considered as mesh 50 and the particles passing through the sieve 140 and remaining on the sieve 400 as mesh 140. Then, they were transferred to containers to prevent moisture absorption. The present study was performed on synthetic sewage containing 25 mg/L of Acid Orange 3. The effective factors studied in this study include the size of the adsorbent particles (eggshell), contact time, adsorbent amount, pH, color concentration, and temperature. For mixing and providing proper contact between adsorbent with color, the shaker (Model: Oxitop IS6) was used at 80 rpm. Separation of adsorbent particles from synthetic sewage solution was performed using a centrifuge (Behdad model) at 4000 rpm for 10 minutes. According to the maximum absorption wavelength for the dye, in this study, the concentration of the dye was measured using a standard curve prepared at concentrations of 2, 5, 10, 20, 40, 50, 80, and 100 mg/L, which was performed using UV/Visible spectrophotometer (model: Unico 2100) at 496 nm wavelength using 1-cm cell.
Acid Orange 3 used in this study was made by Dye Star (Germany) with a molecular weight of 452.39 g/mol and the chemical formula of C18H13N7O7SNa (Fig. 1). Other materials used in the tests were made by Merck (Germany).
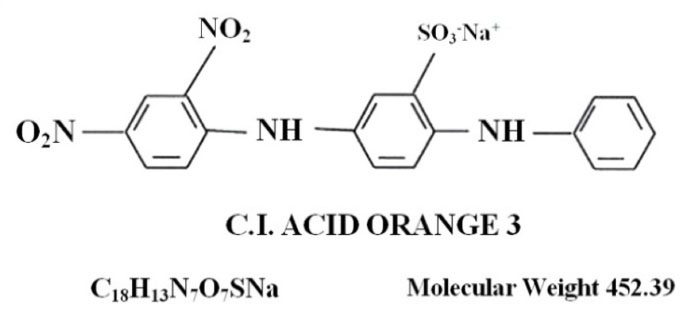
Figure 1.
Specifications of Acid Orange 3.
.
Specifications of Acid Orange 3.
Adsorption Experiments
To evaluate the effect of pH, the pH values of 5, 7, 9, and 11 were measured. Contact times of 30, 60, 90, 120, 150, and 120 minutes were measured for the effect of contact time. The effect of temperature was measured in the 3 ranges of 20, 30, and 40°C. Concentrations of 25, 50, 75, 100, and 200 mg/L of dye were measured based on similar studies (21). A mixing machine was used for mixing at 100 rpm. To investigate the effect of pH, sulfuric acid and caustic soda were used to adjust the pH in different ranges. To measure pH, a pH meter made in Germany (Inolab pH 720 model) was used. To increase the accuracy, all experiments were performed at the laboratory temperature (about 25°C) and experiments were repeated 3 times (N = 74). The average of the data was used for data analysis and Microsoft Excel 2020 was used to draw the charts.
To calculate Acid Orange 3 removal efficiency, Equation 1 was used (15):
Moreover, the determination of absorption isotherm was done using the equations of Freundlich and Langmuir Isotherm (15) as follows:
3. Results and Discussion
3.1. Effect of Adsorbent Dosage
The adsorbent dose was considered as a variable in this study. In this study, the effect of adsorbent mass in the range of 1, 2, 3, 4, and 5 g and the size of adsorbent particles in two mesh sizes of 50 and 140 were investigated in the removal of Acid Orange 3. Fig. 2 shows the effect of these two factors on the removal of the desired dye. Under the same conditions, the percentage of removal of Acid Orange 3 by eggshell increases with smaller particle sizes. Moreover, in both types of grading, by increasing the adsorbent mass from 1 to 5 g, the removal of dye by the adsorbent increases. The results of this study show that by increasing the amount of adsorbent, Acid Orange 3 removal increased so that in constant conditions for adsorbent masses of 2 and 5 g, the dye removal efficiency was calculated to be 36.6% and 55.36%, respectively. This phenomenon is related to an increase in the available surface area for absorbing contaminants (due to an increase in adsorption surface and access to attracting sites) (21). The results of this study show that by reducing the particle size, the amount of Acid Orange 3 removal increased. This phenomenon is attributed to the increase in contact surface of the adsorbent, that is, the smaller the adsorbent particles are, because of the increase in the contact surface, the greater the absorption rate will be (20,21).
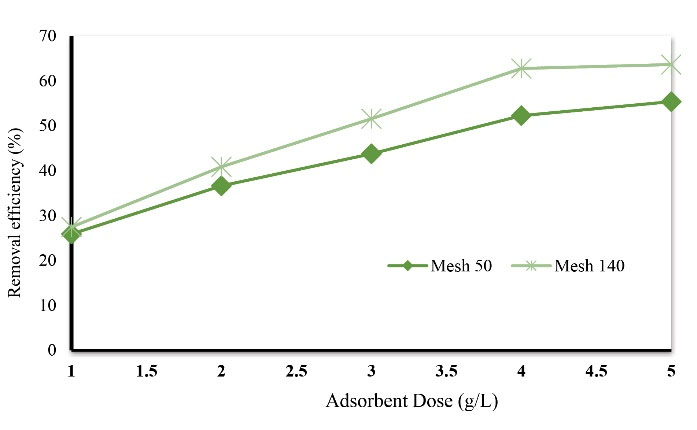
Figure 2.
The Effect of Adsorbent Dosage on Acid Orange 3 Removal Efficiency (initial dye concentration: 25 mg/L, pH: 7, temperature: 25°C, and contact time: 180 minutes).
.
The Effect of Adsorbent Dosage on Acid Orange 3 Removal Efficiency (initial dye concentration: 25 mg/L, pH: 7, temperature: 25°C, and contact time: 180 minutes).
3.2. Effect of Contact Time
Contact time is an important variable in the process of absorption. The absorption capacity and the efficiency of removing dye by the adsorbent have a direct relationship with the contact time. In this study, the effect of time (in 6 ranges of 30, 60, 90, 120, 150, and 180) on the removal of Acid Orange 3 was investigated. Fig. 3 shows the effect of contact time on dye removal. According to Fig. 3, with an increase in contact time with dye solution, the dye removal rate increases so that the maximum removal rate was achieved at a contact time of 180 minutes, and the dye removal rate at this time is equal to 72.1%.
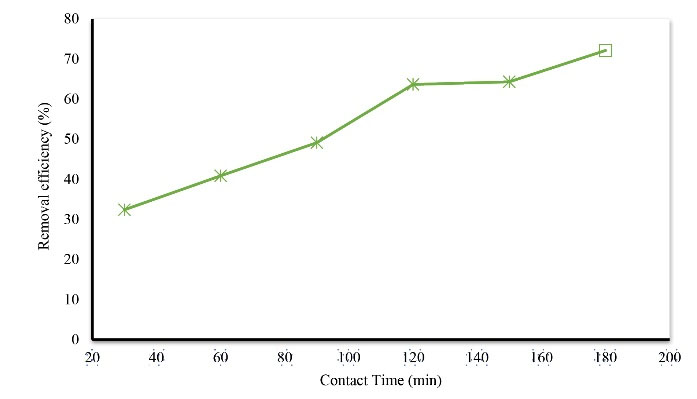
Figure 3.
The Effect of Contact Time on Acid Orange 3 Removal Efficiency 3 (initial dye concentration: 25 mg/L, pH: 7, temperature: 25°C, and adsorbent dosage 5 g/L).
.
The Effect of Contact Time on Acid Orange 3 Removal Efficiency 3 (initial dye concentration: 25 mg/L, pH: 7, temperature: 25°C, and adsorbent dosage 5 g/L).
Regarding the contact time, the results of this study show that although by increasing contact time, the removal time of the dye increased; however, the highest absorption rate occurred within the first 90 minutes. Moreover, these results show that during the absorption of this dye, thin-layer diffusion, which is the first step of absorption, occurred more rapidly. However, penetration in the pores, which leads to increased absorption at the internal surface of the adsorbent, occurred with a delay in which the delay was about 90 minutes for this adsorbent and pollutant. Under constant conditions (concentration of 25 mg/L, temperature of 25°C, neutral pH, and adsorbent dose of 5 g/L), the dye removal efficiency for contact times of 90 and 180 minutes were 49.1% and 72.1%. In a study by Ghaneian et al on the removal of reactive red 123 from synthetic textile sewage using eggshells, the results showed that as the contact time increased, the dye removal efficiency increased and the highest dye absorption occurred in the first 60 minutes (20,21).
3.3. Effect of Solution pH on the Removal Efficiency of Acid Orange 3
One of the most important factors affecting the ionization of pollutants and surface charge of adsorbents is the pH of the reaction medium. In this study, the effect of pH (in the 3 ranges of 5, 7, 9, and 11) on the removal of Acid Orange 3 was investigated. Fig. 4 shows the effect of pH on Acid Orange 3 removal. According to Fig. 5, by increasing pH, the desired dye removal rate increased so that by increasing the pH from 5 to 9, the percentage of dye removal increases from 62.87 to 78.47, respectively. Therefore, the pH value of 9 was considered as the optimal pH for the desired dye removal.
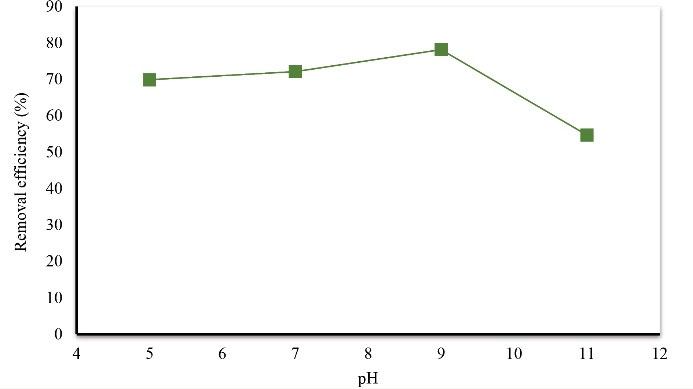
Figure 4.
The Effect of
Contact Time
on Acid Orange 3 Removal Efficiency (initial dye concentration: 25 mg/L, temperature: 25°C, adsorbent dosage: 5 g/L, and contact time: 180 minutes).
.
The Effect of
Contact Time
on Acid Orange 3 Removal Efficiency (initial dye concentration: 25 mg/L, temperature: 25°C, adsorbent dosage: 5 g/L, and contact time: 180 minutes).
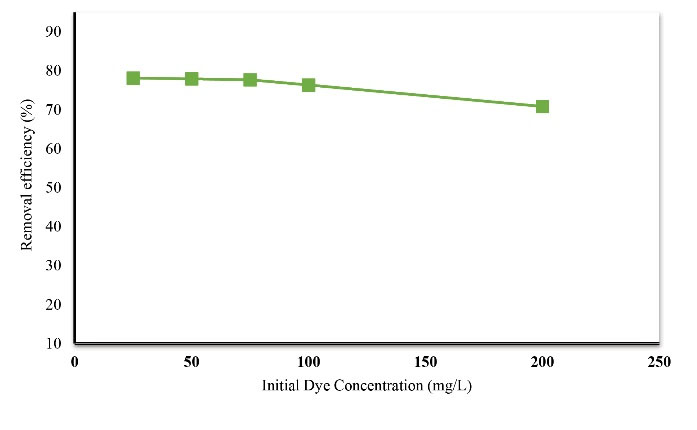
Figure 5.
The Effect of Initial Concentration on Acid Orange 3 Removal Efficiency (pH: 7, temperature: 25°C, adsorbent dosage: 5 g/L, and contact time: 180 minutes).
.
The Effect of Initial Concentration on Acid Orange 3 Removal Efficiency (pH: 7, temperature: 25°C, adsorbent dosage: 5 g/L, and contact time: 180 minutes).
The results related to the effect of pH changes on the dye removal rate indicate that pH is a parameter that influences the amount of dye absorption by eggshells. The results showed that by increasing pH from 5 to 9 and alkalization of the system, the dye removal efficiency increased from 69.87% to 78.14%. This increase is likely to be related to changes in the charge load of the adsorbent surface, which is a function of the pH of the medium (21). However, when the pH increased to 11, the removal efficiency reduced again to about 54.69%. Ghaneian et al showed that by increasing pH from 5 to 8 and alkalizing the system, the efficiency of dye absorption increased from 30 to 48% (21). In a study by Liao et al on the removal of lead from aqueous solutions using hydroxyapatite extracted from eggshells, it has been shown that by increasing pH from 1 to 9, the removal rate of lead increases (24).
3.4. Effect of Initial Concentration
To determine the effect of the amount of dye on the adsorption process the amounts of adsorbent dose, contact time, and pH are considered constant. Concentrations of 25, 50, 75, 100, and 200 mg/L of dye were prepared and the effect of dye concentration on the removal of Acid Orange 3 was investigated. Fig. 5 shows the effect of dye concentration on the desired dye removal. According to Fig. 6, with the increase of the initial dye concentration, the removal efficiency of the adsorbent reduced. Therefore, the maximum removal at the concentration of 25 mg/L was 78.24%, by increasing the dye concentration to 200 mg/L, the dye removal rate reduced to 70%.
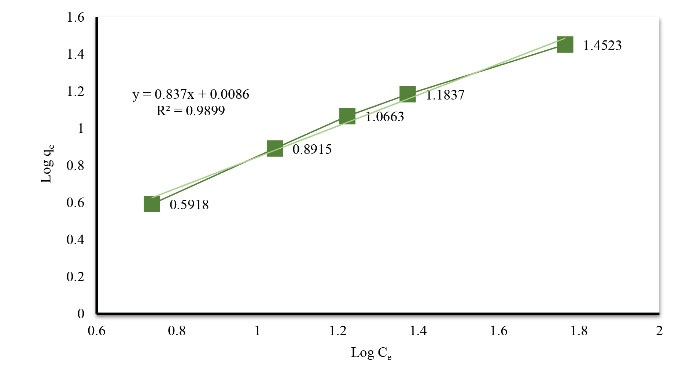
Figure 6.
Freundlich Isotherm Model for the Adsorption of Acid Orange 3.
.
Freundlich Isotherm Model for the Adsorption of Acid Orange 3.
Based on the results of this study, it was found that by increasing initial dye concentration, the removal efficiency of the dye decreases, as the initial dye concentration increased from 25 to 200 mg/L, the dye removal efficiency decreased from 78.14% to 70.83%. This decrease in the rate of absorption by increasing the initial concentration of dye can be attributed to the decrease in available absorption surface due to saturation of adsorption sites (21). Similar results have been reported by other researchers. In a study by Shokohi et al on the removal of reactive black 5 from the aqueous media, through absorption on the Magnetic beads of synthesized sodium alginate, it was shown that by increasing the initial concentration of the dye, the removal efficiency rate significantly reduced (25). In a study by Mahvi and Heibati, activated carbon was prepared from walnut wood and its efficiency in the removal of azo dyes from textile wastewater was assessed (26). The results showed that by increasing initial dye concentration from 25 mg/L to 100 mg/L, the Acid Red 18 removal efficiency decreased from 56.56% to 28.02%. This is due to the number of stationary positions on a given amount of the adsorbent (26).
3.5. Adsorption Isotherm Models
To investigate the adsorption isotherm, experiments were performed using 25 mg/L dye concentration as an initial concentration using various adsorbent dosages (1–5 g/L) at pH 9 for 72 hours. The results of laboratory data analysis showed that Acid Orange 3 adsorption followed Freundlich isotherm model (Fig. 6). Freundlich isotherm constant of Acid Orange 3 by eggshell was compared with that by other adsorbents in Table 1.
Table 1.
Freundlich Isotherm Constants for the Adsorption of Dyes by Different Adsorbents
Temperature
(°K)
|
Adsorbent
|
Dyes
|
Concentration
(mg/L)
|
Freundlich Constants
|
Reference
|
K
F
(mg
1_1/n
L
1/n
g
_1
)
|
n
|
R
2
|
| 293 |
Pumice |
RB5 |
20 |
0.191 |
1.17 |
0.991 |
27 |
| - |
Modified red mud |
Acid Orange 7 |
20 |
1.34 |
28 |
0.986 |
28 |
| 298 |
Eggshell |
Acid Orange 3 |
25 |
1.01 |
1.9 |
0.989 |
This work |
4. Conclusion
It can be concluded that eggshell can be very effectively employed for the removal of Acid Orange 3 from aqueous solution. The adsorption process is highly influenced by various factors such as contact time, initial concentration, adsorbent dose, temperature, and pH. The results show that eggshell has the best effect at pH = 9 and by increasing the adsorbent dose and reducing the size of the adsorbent particles, the removal rate of the dye increases. The results also showed that by increasing temperature and time of contact, the removal rate of dye increased, while by increasing dye concentration, the removal efficiency decreased. Freundlich isotherm model was found to be the best fitted model for the adsorption process.
Conflict of Interest Disclosures
The authors have no conflict of interests to declare with regard to this study.
Acknowledgments
The authors wish to thank Sabzevar University of Medical Sciences, Iran, for the financial support received for this project.
References
- Kaur S, Singh V. TiO2 mediated photocatalytic degradation studies of Reactive Red 198 by UV irradiation. J Hazard Mater 2007; 141(1):230-6. doi: 10.1016/j.jhazmat.2006.06.123 [Crossref] [ Google Scholar]
- Lucas MS, Peres JA. Degradation of Reactive Black 5 by Fenton/UV-C and ferrioxalate/H2O2/solar light processes. Dyes Pigm 2007; 74(3):622-9. doi: 10.1016/j.dyepig.2006.04.005 [Crossref] [ Google Scholar]
- dos Santos AB, Cervantes FJ, van Lier JB. Review paper on current technologies for decolourisation of textile wastewaters: perspectives for anaerobic biotechnology. Bioresour Technol 2007; 98(12):2369-85. doi: 10.1016/j.biortech.2006.11.013 [Crossref] [ Google Scholar]
- Attia AA, Girgis BS, Fathy NA. Removal of methylene blue by carbons derived from peach stones by H3PO4 activation: batch and column studies. Dyes Pigm 2008; 76(1):282-9. doi: 10.1016/j.dyepig.2006.08.039 [Crossref] [ Google Scholar]
- Banat F, Al-Asheh S, Al-Ahmad R, Bni-Khalid F. Bench-scale and packed bed sorption of methylene blue using treated olive pomace and charcoal. Bioresour Technol 2007; 98(16):3017-25. doi: 10.1016/j.biortech.2006.10.023 [Crossref] [ Google Scholar]
- Daneshvar N, Salari D, Khataee AR. Photocatalytic degradation of azo dye Acid Red 14 in water: investigation of the effect of operational parameters. J Photochem Photobiol A Chem 2003; 157(1):111-6. doi: 10.1016/s1010-6030(03)00015-7 [Crossref] [ Google Scholar]
- Nilsson I, Möller A, Mattiasson B, Rubindamayugi MST, Welander U. Decolorization of synthetic and real textile wastewater by the use of white-rot fungi. Enzyme Microb Technol 2006; 38(1):94-100. doi: 10.1016/j.enzmictec.2005.04.020 [Crossref] [ Google Scholar]
- Mohorčič M, Teodorovič S, Golob V, Friedrich J. Fungal and enzymatic decolourisation of artificial textile dye baths. Chemosphere 2006; 63(10):1709-17. doi: 10.1016/j.chemosphere.2005.09.063 [Crossref] [ Google Scholar]
- Tang C, Chen V. The photocatalytic degradation of reactive black 5 using TiO2/UV in an annular photoreactor. Water Res 2004; 38(11):2775-81. doi: 10.1016/j.watres.2004.03.020 [Crossref] [ Google Scholar]
- Naddafi K, Nabizadeh Nodehi R, Jahangiri Rad M. Removal of reactive blue 29 dye from water by single-wall carbon nanotubes. Iran J Health Environ 2011; 3(4):359-68. [ Google Scholar]
- Sahin EM, Tongur T, Ayranci E. Removal of azo dyes from aqueous solutions by adsorption and electrosorption as monitored with in-situ UV-visible spectroscopy. Sep Sci Technol 2020; 55(18):3287-98. doi: 10.1080/01496395.2019.1676786 [Crossref] [ Google Scholar]
- López C, Valade AG, Combourieu B, Mielgo I, Bouchon B, Lema JM. Mechanism of enzymatic degradation of the azo dye Orange II determined by ex situ 1H nuclear magnetic resonance and electrospray ionization-ion trap mass spectrometry. Anal Biochem 2004; 335(1):135-49. doi: 10.1016/j.ab.2004.08.037 [Crossref] [ Google Scholar]
- Benkli YE, Can MF, Turan M, Çelik MS. Modification of organo-zeolite surface for the removal of reactive azo dyes in fixed-bed reactors. Water Res 2005; 39(2):487-93. doi: 10.1016/j.watres.2004.10.008 [Crossref] [ Google Scholar]
- Babu BR, Parande AK, Raghu S, Kumar TP. Cotton textile processing: waste generation and effluent treatment. J Cotton Sci 2007; 11(3):141-53. [ Google Scholar]
- Ong ST, Lee CK, Zainal Z. Removal of basic and reactive dyes using ethylenediamine modified rice hull. Bioresour Technol 2007; 98(15):2792-9. doi: 10.1016/j.biortech.2006.05.011 [Crossref] [ Google Scholar]
- Tsai WT, Hsien KJ, Hsu HC, Lin CM, Lin KY, Chiu CH. Utilization of ground eggshell waste as an adsorbent for the removal of dyes from aqueous solution. Bioresour Technol 2008; 99(6):1623-9. doi: 10.1016/j.biortech.2007.04.010 [Crossref] [ Google Scholar]
- Dong Q, Su H, Zhang D, Liu Z, Lai Y. Synthesis of hierarchical mesoporous titania with interwoven networks by eggshell membrane directed sol–gel technique. Microporous Mesoporous Mater 2007; 98(1-3):344-51. doi: 10.1016/j.micromeso.2006.09.041 [Crossref] [ Google Scholar]
- Abdel-Salam ZA, Abdou AM, Harith MA. Elemental and ultrastructural analysis of the eggshell: Ca, Mg and Na distribution during embryonic development via LIBS and SEM techniques. Int J Poult Sci 2006; 5(1):35-42. doi: 10.3923/ijps.2006.35.42 [Crossref] [ Google Scholar]
- Tsai WT, Yang JM, Lai CW, Cheng YH, Lin CC, Yeh CW. Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour Technol 2006; 97(3):488-93. doi: 10.1016/j.biortech.2005.02.050 [Crossref] [ Google Scholar]
- Tsai WT, Hsien KJ, Hsu HC, Lin CM, Lin KY, Chiu CH. Utilization of ground eggshell waste as an adsorbent for the removal of dyes from aqueous solution. Bioresour Technol 2008; 99(6):1623-9. doi: 10.1016/j.biortech.2007.04.010 [Crossref] [ Google Scholar]
- Ghaneian MT, Ghanizadeh G, Gholami M, Ghaderinasab F. Application of eggshell as a natural sorbent for the removal of reactive red 123 dye from synthetic textile wastewater. Zahedan Journal of Research in Medical Sciences 2010; 11(4):25-34. [ Google Scholar]
- Zheng W, Li XM, Yang Q, Zeng GM, Shen XX, Zhang Y. Adsorption of Cd(II) and Cu(II) from aqueous solution by carbonate hydroxylapatite derived from eggshell waste. J Hazard Mater 2007; 147(1-2):534-9. doi: 10.1016/j.jhazmat.2007.01.048 [Crossref] [ Google Scholar]
- Tan CY, Li M, Lin YM, Lu XQ, Chen ZL. Biosorption of Basic Orange from aqueous solution onto dried A filiculoides biomass: equilibrium, kinetic and FTIR studies. Desalination 2011; 266(1-3):56-62. doi: 10.1016/j.desal.2010.08.002 [Crossref] [ Google Scholar]
- Liao D, Zheng W, Li X, Yang Q, Yue X, Guo L. Removal of lead(II) from aqueous solutions using carbonate hydroxyapatite extracted from eggshell waste. J Hazard Mater 2010; 177(1-3):126-30. doi: 10.1016/j.jhazmat.2009.12.005 [Crossref] [ Google Scholar]
- Shokohi R, Samarghandi MR, Pourfarzi F, Shirzad Siboni M, Vahedi H. Removal of Reactive Black 5 (RB5) dye from aquatic solution by using of adsorption onto synthesized sodium alginate magnetic beads. Iran J Health Environ 2011; 4(1):1-10. [ Google Scholar]
- Mahvi AH, Heibati B. Removal efficiency of azo dyes from textile effluent using activated carbon made from walnut wood and determination of isotherms of Acid Red 18. J Health 2010; 1(3):7-15. [ Google Scholar]
- Heibati B, Rodriguez-Couto S, Amrane A, Rafatullah M, Hawari A, Al-Ghouti MA. Uptake of Reactive Black 5 by pumice and walnut activated carbon: chemistry and adsorption mechanisms. J Ind Eng Chem 2014; 20(5):2939-47. doi: 10.1016/j.jiec.2013.10.063 [Crossref] [ Google Scholar]
- Zazouli MA, Belarak D, Mahdavi Y. Application of modified red mud for adsorption of Acid Orange 7 (AO7) dye from aqueous solution: isotherms, kinetics studies. J Health Res Community 2015; 1(2):1-11. [ Google Scholar]