Avicenna J Environ Health Eng. 7(1):8-13.
doi: 10.34172/ajehe.2020.02
Original Article
Solid Phase Extraction Method for Separation and Pre-concentration of Thallium in Water Samples Using a Novel Adsorbent
Mohammad Reza Ansari 1, Ali Sheibani 1, *  , Masoumeh Tabatabaee 1
, Masoumeh Tabatabaee 1
Author information:
1Department of Chemistry, Yazd Branch, Islamic Azad University, Yazd, Iran.
*Correspondence to Ali Sheibani, Tel: +98-35-31872585, Fax: +98-35-38214810 Email: sheibani@iauyazd.ac.ir,
alisheibani@ymail.com
Abstract
In this study, a new and simple column-mode separation method has been proposed for the pre-concentration and removal of trace amounts of thallium(III) (Tl) from water samples. The procedure was based on sorption of Tl(III) ions by a solid phase extraction column filled with 4-[(2-hydroxy, 3-methoxy benzylidine)4-amino]1,2,4-treeazol-5-thione on microcrystalline naphthalene. The determination was performed directly using flame atomic absorption spectrometry (FAAS) method. The main factors affecting the extraction recovery were investigated and optimized. The interference effect of various ions on the determination of Tl(III) was also studied. In optimum conditions, the precision of the proposed method for sample solutions containing 0.2 µg/mL of Tl(III) was 2.0%, and the enrichment factor was found to be 35. The proposed method was evaluated and employed for pre-concentration and removal of Tl(III) from spiked natural (Tap and well) and industrial water samples.
Keywords: Pre-concentration, Thallium (Tl), Solid phase extraction (SPE), Removal, Flame atomic absorption spectrometry (FAAS), Water samples
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
Among all the pollutants, heavy metals have received considerable attention owing to their role in the pollution of the environment. Thallium (Tl) as a heavy metal has been identified to be an environmentally important element due to its toxic effects. It is regarded as one of the most toxic heavy metals causing both chronic and acute poisoning. In the long term, Tl has the potential to cause changes in blood chemistry, damage to liver, kidney, intestinal and testicular tissues, and hair loss. Generally, it exists in nature as Tl(I) and Tl(III) ions that exhibit different bioavailability and toxicity properties. It has been stated that Tl(III) is more toxic than Tl(I). Tl exists in the environment commonly together with lead, zinc, iron, tellurium, and alkalis. It is used as a catalyst in making alloys, optical lenses, low temperature thermometers, dyes, pigment in scintillation counters. Additionally, it is used as medicine, rodenticide, and insecticide (1-5). Therefore, considerable interest in sample preparation and determination of Tl has been seen in the research literature.
Various analytical procedures have been presented for pre-concentration (extraction) and removal of Tl, mainly based on liquid-liquid extraction and dispersive liquid-liquid extraction (6-8), single drop liquid-phase micro-extraction (9), solid-phase extraction (SPE) and dispersive SPE (10-15), co-precipitation and ion-exchange (16-19).
Among these techniques, SPE is one of the most effective procedures because of its simplicity, rapidity, and capability to reach a high enrichment factor. Furthermore, SPE can eliminate the interfering matrix effects and also has the ability to combine with some detection methods.
In SPE technique, the sorbent is a critical factor to obtain suitable recovery and selectivity. In the present paper, a simple and accurate pre-concentration methodology, based on sorption of Tl(III) by an SPE column filled with 4-[(2-hydroxy, 3-methoxy benzylidine)4-amino]1,2,4-tree azol-5-thione on microcrystalline naphthalene as a new sorbent, has been established. The determination process was directly carried out by flame atomic absorption spectrometry (FAAS). The influence of various factors in the adsorption and removal of Tl(III) from sample solutions was investigated and optimized. Finally, the practical capability of the proposed method was tested using the spiked natural and industrial water samples.
2. Materials and Methods
2.1. Instrumentation
FAAS (Varian Spectra 220, Australia) equipped with D2 lamp for background correction and air-acetylene burner was used for absorbance measurements. Hollow cathode lamp was employed as a radiation source at the wavelength of 276.8 nm (12). The operating parameters (10 mA and bandwidth 0.5 nm) were according to the manufacturer’s recommendation. In order to obtain the maximum absorbance signal, the acetylene flow rate and burner height were also adjusted. A pH meter (ISTEK-720P, South Korea) was used for measuring the acidity of sample solutions.
2.2. Chemicals and Standards
Chemicals and solvents from Merck were used. A stock solution of 100 µg/mL of Tl(III) was prepared by dissolving an accurate weight of Tl(NO3)3·3H2O (98%) in distilled water in a 100 mL flask and diluting to the mark with solvent. Working solutions were prepared by appropriate dilution of it with distilled water. The Excel software program (2010) was used for statistical analysis.
2.3. Preparation of SPE Cartridge
4-[(2-hydroxy, 3-methoxy benzylidine)4-amino]1,2,4-treeazol-5-thione was synthesized according to the literature procedure (20) using 2-hydroxy-3-methoxybenzaldhyde as aldehyde. The solution of 4-amino-5-methyl-2H-1,2,4-triazole-3(4H)-thione (in C2H5OH) was treated with 2-hydroxy-3-methoxybenzaldehyde in a molar ratio of 1:1.2 and the resulting mixture was acidified by hydrochloric acid (37%, 4 drops). The reaction mixture was refluxed for 10 hours. The solid residue was filtered and washed with cold solvent. The molecular structure of ligand is shown in Fig. 1.
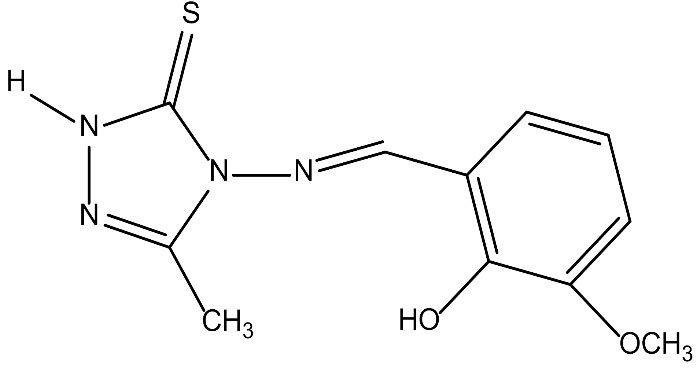
Figure 1.
The Molecular Structure of Ligand:E)-4-((2-hydroxy-3-methoxybenzylidene)amino)-3-methyl-1H-1,2,4-triazole-5(4H)-thione.
.
The Molecular Structure of Ligand:E)-4-((2-hydroxy-3-methoxybenzylidene)amino)-3-methyl-1H-1,2,4-triazole-5(4H)-thione.
A mixture containing 20.0 g of naphthalene, 2.0 g of the synthesized ligand (as adsorbent), and 60 mL of acetone was prepared in a 500 mL beaker. It was mixed at 40oC for 20 minutes. Then, distilled water was gradually added and the solution was stirred again for 30 minutes. The solid mass was separated using a Whatman filter paper, dried, and washed by distilled water.
The prepared adsorbent was used as a solid matrix material in a homemade SPE cartridge. The SPE column was a plastic syringe with a diameter of 9 mm and a length of 9 cm. The bottom of the column was plugged with Teflon wool to prevent loss of the adsorbent during the SPE process. Then, it was filled up and packed without gaps by adding 0.20 g of adsorbent and placing a stopcock at the upper end. The length of adsorbent inserted in the column was about 50 mm.
2.4. Pre-concentration and Determination of Tl( III)
Working solutions containing Tl(III) were prepared and their pH values were adjusted using the HNO3 or NaOH solutions (0.1 mol/L). The prepared solutions (50 mL) were passed through the column with an appropriate flow rate. In the next step, the column was washed with distilled water to remove the effect of matrix interferences for Tl(III) measurement. Then, the adsorbed analyte ions on the SPE column were separated and eluted using HNO3. Finally, the Tl(III) determination was performed by FAAS method at the wavelength of 276.8 nm and in optimum operating conditions. The schematic diagram of the flow system used for pre-concentration and determination of Tl(III) is shown in Fig. 2.
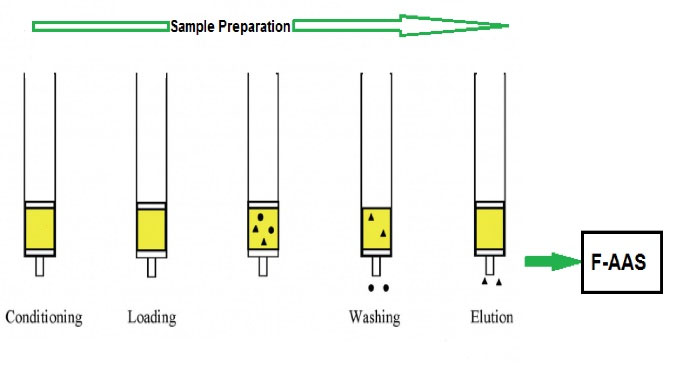
Figure 2.
The Schematic Diagram of Pre-concentration and Determination Steps for Tl(III) Using the Proposed Method.
.
The Schematic Diagram of Pre-concentration and Determination Steps for Tl(III) Using the Proposed Method.
2.5. SPE of Different Spiked Water Samples
Tap and well water samples were obtained from the laboratory and the neighboring areas of the Islamic Azad University of Yazd, respectively. The samples were collected after flowing for a few minutes in 1000 mL PE bottles. Industrial water (used in combined cycle power plant for purposes such as processing, washing, and cooling) samples were prepared from several sites of Yazd power plant and collected in 1000 mL PE bottles. The water samples were stored at 4°C in a refrigerator until analysis. The synthetic samples were prepared and filtered through a 0.45 μm filter to remove any suspended materials and were treated according to the proposed procedure.
3. Results and Discussion
In order to obtain a better extraction recovery, the effects of various factors on SPE including pH, eluent (type, concentration, and volume), and sample solution (flow rate and volume) were investigated and optimized by one-factor-at-a-time method. The extraction recovery of Tl(III) was calculated based on the following formula:
3.1. Effect of pH
The pH value of the sample solution plays an important role in the adsorption process (12) of the Tl(III) on the prepared SPE column. The effect of pH on the extraction recovery of Tl(III) was investigated in the range of 1–10, according to the proposed procedure. The results are shown in Fig. 3, which indicated that the maximum recovery of Tl(III) (87.7%) occurs at pH=8 and then it decreased. The low extraction recovery at the acidic pH values is due to the competition of proton with Tl(III) for adsorption on the adsorbent and also at the basic pH values, Tl(III) can precipitate as Tl(OH)3. Therefore, pH=8.0 was selected as the optimum value in the following experiments.
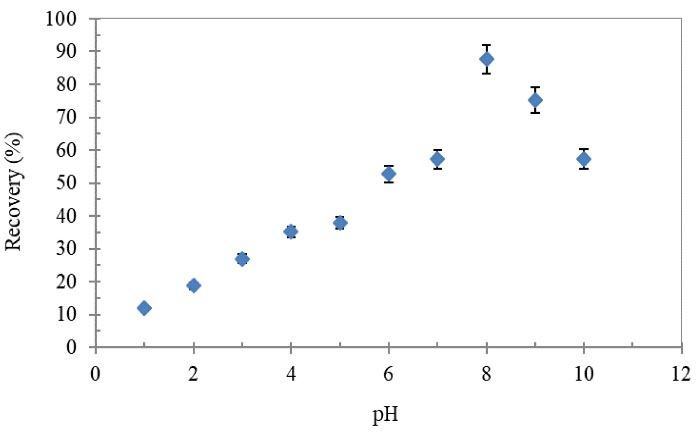
Figure 3.
Effect of pH on Recovery of Tl(III) Ions Using the Proposed SPE Column
Conditions: Tl(III) concentration: 0.2 µg/mL; sample volume: 40 mL; sample flow rate: 4 mL/min; HNO3 (1 mol/L, 3 mL).
.
Effect of pH on Recovery of Tl(III) Ions Using the Proposed SPE Column
Conditions: Tl(III) concentration: 0.2 µg/mL; sample volume: 40 mL; sample flow rate: 4 mL/min; HNO3 (1 mol/L, 3 mL).
3.2. Effect of Type, Concentration, and Volume of Elution Solvent
The eluent is another important factor to be considered for obtaining quantitative extraction recovery in SPE studies (21). It must be able to desorb the analyte ions in a low volume while being compatible with the determination method (FAAS). In order to choose the best eluent among the potential solvents, under obtained conditions, different solvents such as nitric acid, phosphoric acid, and ammonia were examined and their extraction recoveries were 90.6%, 47.4%, and 15.2%, respectively. Therefore, it was found that nitric acid can provide the complete desorption and best extraction recovery.
The effect of nitric acid concentration (0.5-3.0 mol/L) on the extraction recovery of Tl(III) ions from aqueous solutions was also tested (Fig. 4). According to the results shown in Fig. 4, the best extraction recovery (>90%) for HNO3 was achieved at the concentration of 3.0 mol/L. Further increases in nitric acid concentration can lead to the destruction of adsorbent structure and decreased extraction recovery. Therefore, 3.0 mol/L was used for subsequent investigations.
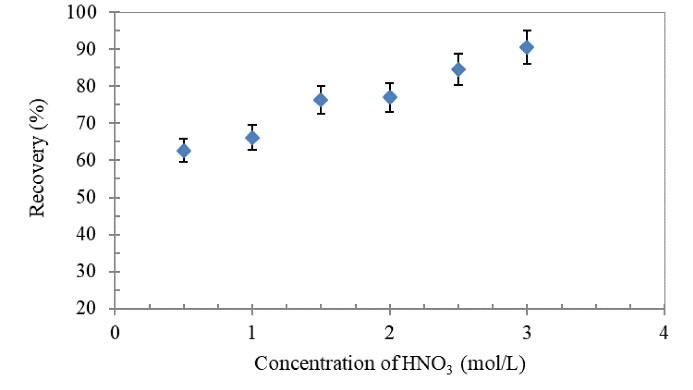
Figure 4.
Effect of Concentration of HNO3 (Eluent, 3 mL)on Recovery of Tl(III) Ions Using the Proposed SPE Column.
.
Effect of Concentration of HNO3 (Eluent, 3 mL)on Recovery of Tl(III) Ions Using the Proposed SPE Column.
Conditions: Tl(III) concentration: 0.2 µg/mL; sample volume: 40 mL; sample flow rate: 4 mL/min; pH: 8.
Different volumes (0.5-5 mL) of nitric acid (3.0 mol/L) were examined for elution of adsorbed Tl(III) from homemade SPE cartridge. The experimental results are depicted in Fig. 5. As it is seen, the extraction recovery of metal ions increased up to a volume of 1.5 mL and then it reached a constant level. Therefore, 1.5 mL of nitric acid (3.0 mol/L) was found to be a sufficient value for both quantitative recovery of the analyte ions and solvent saving.
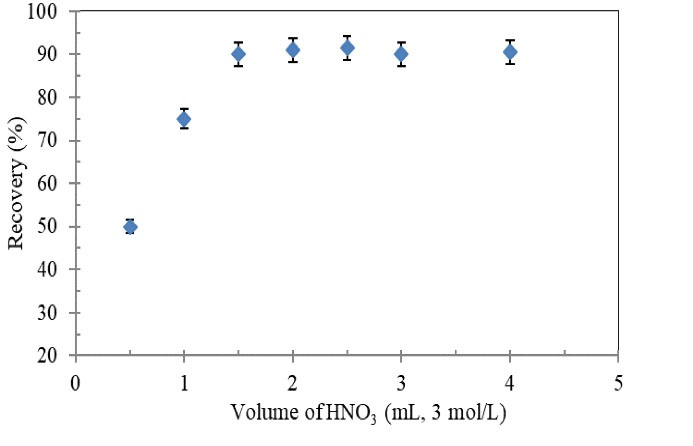
Figure 5.
Effect of Volume of HNO3 (eluent)on Recovery of Tl(III) Ions Using the Proposed SPE Column.
Conditions: Tl(III) concentration: 0.2 µg/mL; sample volume: 40 mL; sample flow rate: 4 mL/min; pH: 8; HNO3 (3 mol/L).
.
Effect of Volume of HNO3 (eluent)on Recovery of Tl(III) Ions Using the Proposed SPE Column.
Conditions: Tl(III) concentration: 0.2 µg/mL; sample volume: 40 mL; sample flow rate: 4 mL/min; pH: 8; HNO3 (3 mol/L).
3.3. Effect of Sample (Flow Rate and Volume)
The sample flow rate through SPE column has a significant effect on both the analyte adsorption and analysis time (22) The influence of this factor on the extraction recovery was investigated at different flow rate values (1–8 mL/min) (Fig. 6). As it is seen in Fig. 6, the flow rate in the range of 1–6 mL/min had a significant effect on the recovery of Tl(III) while at higher values, it decreased. This can be attributed to incomplete adsorption of Tl(III) ions on the column. Therefore, the flow rate of 6 mL/min was adopted to obtain the maximum recovery of Tl(III) ions and save time.
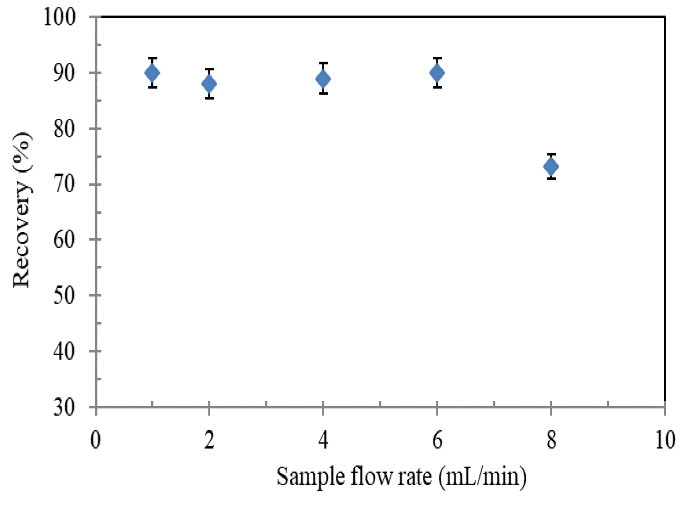
Figure 6.
Effect of Sample Flow Rateon Recovery of Tl(III) Ions Using the Proposed SPE Column
Conditions: Tl(III) concentration: 0.2 µg/mL; sample volume: 40 mL; HNO3 (3 mol/L, 1.5 mL), pH: 8.
.
Effect of Sample Flow Rateon Recovery of Tl(III) Ions Using the Proposed SPE Column
Conditions: Tl(III) concentration: 0.2 µg/mL; sample volume: 40 mL; HNO3 (3 mol/L, 1.5 mL), pH: 8.
In order to evaluate the breakthrough volume of the SPE cartridge, a constant amount of analyte was added to different volumes of distilled water (20-200 mL) and was loaded on the prepared column and then was treated based on the proposed procedure. According to the recovery results shown in Fig. 7, volumes of up to 50 mL of sample solution could be applied without significant loss of extraction recovery for Tl(III). Consequently, the enrichment factor of 35 was also achieved in the presented work.
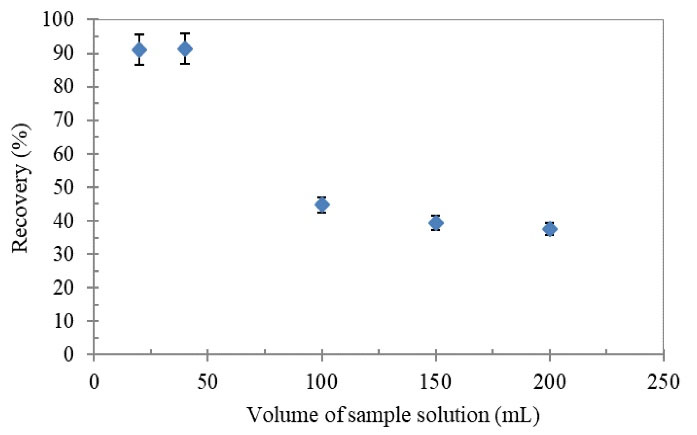
Figure 7.
Effect of Sample Volume on Recovery of Tl(III) Ions Using the Proposed SPE Column
Conditions: Tl(III) concentration: 0.2 µg/mL; sample flow rate: 6 mL/min; pH: 8; HNO3 (3 mol/L, 1.5 mL).
.
Effect of Sample Volume on Recovery of Tl(III) Ions Using the Proposed SPE Column
Conditions: Tl(III) concentration: 0.2 µg/mL; sample flow rate: 6 mL/min; pH: 8; HNO3 (3 mol/L, 1.5 mL).
3.4. Effect of Divers Ions
For selectivity study, the effect of potential interferences from some foreign ions was tested on the recovery of 0.2 µg/mL of Tl(III) using the proposed method. The results are summarized in Table 1. The tolerance limit was defined as the maximum concentration of ions which caused a relative error smaller than ±3% in determination of the analyte ions. According to this Table, most of the ions did not interfere, even when presented in 500 to 1000-fold excess over analyte. Therefore, the proposed method showed a good selectivity that could be applied in pre-concentration (or removal) and determination of Tl(III) in real water samples.
Table 1.
Tolerance Limit for Diverse Ions in the Determination of 0.2 µg/mL of Tl(III)
|
Foreign Ion
|
Tolerance Limit
(w
ion
/w
Tl
)
|
| Na+ |
1000 |
| K+ |
1000 |
| Cl- |
1000 |
| NO3- |
1000 |
| Ca2+ |
750 |
| Mg2+ |
500 |
| Mn2+ |
500 |
| Sn2+ |
100 |
| Fe3+ |
100 |
| Co2+ |
50 |
| Ni2+ |
50 |
| Cu2+ |
10 |
| Ag+ |
5 |
3.5. Application of the Proposed Method for Different Water Samples
In order to evaluate the extraction recovery of the proposed method, it was exploited for sample preparation and determination of Tl(III) from three water samples (well, tap, and industrial water) which have been collected from the local area of Yazd, Iran. The known amounts (2 levels) of the standard solution of Tl(III) were spiked to the water samples. The concentration of Tl(III) in synthetic water samples and their extraction recoveries are listed in Table 2. The values varied within the range of 93.0%–95.0% with RSD of 2-3%. The satisfactory recovery results obtained confirm that the proposed SPE-FAAS is a suitable alternative method for trace determination of Tl(III) in such water samples.
Table 2.
Application Studies for Adsorption of Tl(III) in Different Water Samples Using the Proposed Method
|
Water sample
|
Tl(III)
|
Recovery %
|
|
Added (µg/mL)
|
Found (µg/mL)
|
| Drinking |
- |
- |
- |
|
|
0.10 |
0.094 |
94 ± 1.8 |
|
|
0.20 |
0.19 |
95 ± 2.0 |
| Tap |
- |
- |
- |
|
|
0.10 |
0.093 |
93 ± 1.5 |
|
|
0.20 |
0.19 |
95 ± 2.2 |
| Industriala |
- |
- |
- |
|
|
0.10 |
0.093 |
93 ± 2.5 |
|
|
0.20 |
0.19 |
95 ± 3.0 |
a Combined cycle power plant.
In addition, some analytical parameters of the proposed method were compared with those of other published methods used for the Tl adsorption of different samples (Table 3). As it is shown, the proposed method presented a suitable accuracy that is comparable to other reported methods. Furthermore, low cost, affordability, and simplicity are the other advantages of the presented method over the reported works (9,12,21-28).
Table 3.
Comparison of the Proposed Method with the Other Methods for Pre-concentration and Determination and of Tl
|
Method
|
Enrichment or Pre-concentration Factor
|
Recovery %
|
RSD %
|
Ref.
|
| DLLME-ICP-MS |
- |
96.3-103 |
1.5 |
23 |
| ILDLLME-ETAAS |
100 |
97.3-103 |
5.3 |
24 |
| CPE-ICP-MS |
125 |
99.6-101.5 |
2.3 |
25 |
| UA-CPE-ICP/OES |
160 |
98.5-101 |
1.7-2.8 |
26 |
| DSPE-FAAS |
298 |
95.5-103 |
3.2 |
21 |
| FI-SPE-FAAS |
77 |
95-103.6 |
2.6 |
27 |
| SPE-FAAS |
100 |
98-104 |
1.2 |
28 |
| SDME–ETAAS |
50 |
94-96 |
5.1 |
9 |
| ET-AAS |
100 |
96.5-101 |
4 |
22 |
| FI–SPE–FAAS |
105 |
94-104 |
3.2 |
12 |
| SPE-FAAS |
35 |
93-95 |
2 |
This work |
DLLME: Dispersive liquid-liquid microextraction; ICP: Inductively coupling plasma; MS: Mass spectrometry; IL: Ionic liquid; UA: Ultrasound assisted; CPE: Cloud point extraction; DSPE: dispersive solid phase extraction; FI: Flow injection; SDME: Single drop microextraction; ET: Electrothermal; OES: Optic emission spectrometry; FAAS: Flame atomic absorption spectrometry.
4. Conclusion
In this study, a procedure for the determination of trace amounts of Tl(III) ions was described using FAAS, which applied a new SPE-column ([(2-hydroxy, 3-methoxy benzylidine)4-amino]1,2,4-tree azol-5-thione on microcrystalline naphthalene) for sample preparation. The system was successful in pre-concentrating analyte ions from sample solutions without the need for additional instrumentation. The analytical validation parameters of the proposed method were comparable with other presented methods. Furthermore, it can be considered as a cost-effective and simple method for Tl(III) determination in different water samples.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
Acknowledgements
The authors are thankful to Yazd Branch of the Islamic Azad University for supporting this work.
References
- Lan CH, Lin TS. Acute toxicity of trivalent thallium compounds to Daphnia magna. Ecotoxicol Environ Saf 2005; 61(3):432-5. doi: 10.1016/j.ecoenv.2004.12.021 [Crossref] [ Google Scholar]
- Townshend A. Encyclopedia of Analytical Science. Vol. 9. London: Academic Press; 1995.
- Galván-Arzate S, Santamaría A. Thallium toxicity. Toxicol Lett 1998; 99(1):1-13. doi: 10.1016/s0378-4274(98)00126-x [Crossref] [ Google Scholar]
- Kazantzis G. Thallium in the environment and health effects. Environ Geochem Health 2000; 22(4):275-80. doi: 10.1023/a:1006791514080 [Crossref] [ Google Scholar]
- Cvjetko P, Cvjetko I, Pavlica M. Thallium toxicity in humans. Arh Hig Rada Toksikol 2010; 61(1):111-9. doi: 10.2478/10004-1254-61-2010-1976 [Crossref] [ Google Scholar]
- Mahamuni SV, Wadgaonkar P, Anuse M. Rapid liquid–liquid extraction of thallium(III) from succinate media with 2-octylaminopyridine in chloroform as the extractant. J Serb Chem Soc 2008; 73(4):435-51. doi: 10.2298/jsc0804435m [Crossref] [ Google Scholar]
- Mohammadi SZ, Sheibani A, Abdollahi F, Shahsavani E. Speciation of Tl(III) and Tl(I) in hair samples by dispersive liquid–liquid microextraction based on solidification of floating organic droplet prior to flame atomic absorption spectrometry determination. Arab J Chem 2016; 9:S1510-S5. doi: 10.1016/j.arabjc.2012.03.008 [Crossref] [ Google Scholar]
- Li Y, Peng G, He Q, Zhu H, Al-Hamadani SM. Dispersive liquid-liquid microextraction based on the solidification of floating organic drop followed by ICP-MS for the simultaneous determination of heavy metals in wastewaters. Spectrochim Acta A Mol Biomol Spectrosc 2015; 140:156-61. doi: 10.1016/j.saa.2014.12.091 [Crossref] [ Google Scholar]
- Chamsaz M, Arbab-Zavar MH, Darroudi A, Salehi T. Preconcentration of thallium (I) by single drop microextraction with electrothermal atomic absorption spectroscopy detection using dicyclohexano-18-crown-6 as extractant system. J Hazard Mater 2009; 167(1-3):597-601. doi: 10.1016/j.jhazmat.2009.01.019 [Crossref] [ Google Scholar]
- Biaduń E, Sadowska M, Ospina-Alvarez N, Krasnodębska-Ostręga B. Direct speciation analysis of thallium based on solid phase extraction and specific retention of a Tl(III) complex on alumina coated with sodium dodecyl sulfate. Mikrochim Acta 2016; 183:177-83. doi: 10.1007/s00604-015-1624-3 [Crossref] [ Google Scholar]
- Biata NR, Dimpe KM, Ramontja J, Mketo N, Nomngongo PN. Determination of thallium in water samples using inductively coupled plasma optical emission spectrometry (ICP-OES) after ultrasonic assisted-dispersive solid phase microextraction. Microchem J 2018; 137:214-22. doi: 10.1016/j.microc.2017.10.020 [Crossref] [ Google Scholar]
- Kazantzi V, Anthemidis A. An on-line flow-injection sorbent extraction system coupled with flame atomic absorption spectrometry for thallium determination using a PTFE turning-packed column. Separations 2019; 6(2):22. doi: 10.3390/separations6020022 [Crossref] [ Google Scholar]
- Gugushe AS, Mpupa A, Nomngongo PN. Ultrasound-assisted magnetic solid phase extraction of lead and thallium in complex environmental samples using magnetic multi-walled carbon nanotubes/zeolite nanocomposite. Microchem J 2019; 149:103960. doi: 10.1016/j.microc.2019.05.060 [Crossref] [ Google Scholar]
- Naghizadeh M, Taher MA, Zeidabadi Nejad L, Hassani Moghaddam F. Fabrication, characterization and theoretical investigation of novel Fe3O4@egg-shell membrane as a green nanosorbent for simultaneous preconcentration of Cu(II) and Tl(I) prior to ETAAS determination. Environ Nanotechnol Monit Manag 2018; 10:171-8. doi: 10.1016/j.enmm.2018.06.001 [Crossref] [ Google Scholar]
- Alalwan HA, Abbas MN, Abudi ZN, Alminshid AH. Adsorption of thallium ion (Tl+3) from aqueous solutions by rice husk in a fixed-bed column: experiment and prediction of breakthrough curves. Environ Technol Innov 2018; 12:1-13. doi: 10.1016/j.eti.2018.07.001 [Crossref] [ Google Scholar]
- Liu W, Zhang P, Borthwick AG, Chen H, Ni J. Adsorption mechanisms of thallium(I) and thallium(III) by titanate nanotubes: ion-exchange and co-precipitation. J Colloid Interface Sci 2014; 423:67-75. doi: 10.1016/j.jcis.2014.02.030 [Crossref] [ Google Scholar]
- Tereshatov EE, Boltoeva MY, Folden CM 3rd. Resin ion exchange and liquid-liquid extraction of indium and thallium from chloride media. Solvent Extr Ion Exch 2015; 33(6):607-24. doi: 10.1080/07366299.2015.1080529 [Crossref] [ Google Scholar]
- Soylak M, Saracoglu S, Divrikli U, Elci L. Coprecipitation of heavy metals with erbium hydroxide for their flame atomic absorption spectrometric determinations in environmental samples. Talanta 2005; 66(5):1098-102. doi: 10.1016/j.talanta.2005.01.030 [Crossref] [ Google Scholar]
- Karlsson U, Düker A, Karlsson S. Separation and quantification of Tl(I) and Tl(III) in fresh water samples. J Environ Sci Health A Tox Hazard Subst Environ Eng 2006; 41(7):1155-67. doi: 10.1080/10934520600655747 [Crossref] [ Google Scholar]
- Ghassemzadeh M, Tabatabaee M, Soleimani S, Neumüller B. Synthesis, characterization and crystal structures of new 1,2,4-triazole based Schiff-bases and their copper(I) complexes. Z Anorg Allg Chem 2005; 631(10):1871-6. doi: 10.1002/zaac.200500140 [Crossref] [ Google Scholar]
- Dehghani Firouzabadi Z, Haji Shabania AM, Dadfarnia S, Ehrampoush MH. Preconcentration and speciation of thallium by ferrofluid based dispersive solid phase extraction and flame atomic absorption spectrometry. Microchem J 2017; 130:428-35. doi: 10.1016/j.microc.2016.10.025 [Crossref] [ Google Scholar]
- Fayazi M, Ghanei-Motlagh M, Taher MA, Ghanei-Motlagh R, Salavati MR. Synthesis and application of a novel nanostructured ion-imprinted polymer for the preconcentration and determination of thallium(I) ions in water samples. J Hazard Mater 2016; 309:27-36. doi: 10.1016/j.jhazmat.2016.02.002 [Crossref] [ Google Scholar]
- Escudero LB, Wuilloud RG, Olsina RA. Sensitive determination of thallium species in drinking and natural water by ionic liquid-assisted ion-pairing liquid-liquid microextraction and inductively coupled plasma mass spectrometry. J Hazard Mater 2013; 244-245:380-6. doi: 10.1016/j.jhazmat.2012.11.057 [Crossref] [ Google Scholar]
- Escudero LB, Berton P, Martinis EM, Olsina RA, Wuilloud RG. Dispersive liquid-liquid microextraction and preconcentration of thallium species in water samples by two ionic liquids applied as ion-pairing reagent and extractant phase. Talanta 2012; 88:277-83. doi: 10.1016/j.talanta.2011.09.068 [Crossref] [ Google Scholar]
- Meeravali NN, Jiang SJ. Ultra-trace speciation analysis of thallium in environmental water samples by inductively coupled plasma mass spectrometry after a novel sequential mixed-micelle cloud point extraction. J Anal At Spectrom 2008; 23(4):555-60. doi: 10.1039/b718149c [Crossref] [ Google Scholar]
- Biata NR, Mashile GP, Ramontja J, Mketo N, Nomngongo PN. Application of ultrasound-assisted cloud point extraction for preconcentration of antimony, tin and thallium in food and water samples prior to ICP-OES determination. J Food Compost Anal 2019; 76:14-21. doi: 10.1016/j.jfca.2018.11.004 [Crossref] [ Google Scholar]
- Dadfarnia S, Assadollahi T, Haji Shabani AM. Speciation and determination of thallium by on-line microcolumn separation/preconcentration by flow injection-flame atomic absorption spectrometry using immobilized oxine as sorbent. J Hazard Mater 2007; 148(1-2):446-52. doi: 10.1016/j.jhazmat.2007.02.059 [Crossref] [ Google Scholar]
- Mashhadizadeh MH, Mostafavi A, Allah-Abadi H, Zadmehr MR. Flame atomic absorption spectrometric determination of ultra traces of thallium(I) ion after solid phase extraction by octadecyl silica membrane disk modified by a new Schiff base. Bull Korean Chem Soc 2004; 25(9):1309-13. doi: 10.5012/bkcs.2004.25.9.1309 [Crossref] [ Google Scholar]