Avicenna J Environ Health Eng. 6(2):100-105.
doi: 10.34172/ajehe.2019.13
Original Article
Synthesis, Characterization, and Biological Evaluation of Chromium(III) Complexes of Alanine and Valine
Shuaibu Musa 1, *  , Suleiman Ola Idris 1, David A. Onu 2, Ahmed B. Suleiman 3
, Suleiman Ola Idris 1, David A. Onu 2, Ahmed B. Suleiman 3
Author information:
1Department of Chemistry, Ahmadu Bello University, Zaria, Nigeria.
2Department of Chemistry, Federal College of Education, Zaria, Nigeria.
3Department of Microbiology, Ahmadu Bello University, Zaria, Nigeria.
*Correspondence to Shuaibu Musa, Department of Chemistry, Ahmadu Bello University, Zaria, Nigeria, Tel: +2347030111330, Email:
shuaibumusa24@gmail.com
Abstract
Two metal-amino acid complexes, Cr(III)-alanine and Cr(III)-valine, were synthesized and characterized by IR spectroscopy, powder X-ray diffraction (XRD) analysis, magnetic susceptibility, and molar conductivity measurements. Molar conductivity measurements indicated that the composition of the metal complexes corresponds to a metal-amino acid ligand ratio of 1:3. The IR spectra indicated that the amino acids act as bidentate ligands with coordination involving the carboxyl oxygen and the nitrogen of the amino group. Magnetic susceptibility measurements revealed a six-coordinate local symmetry around the Cr(III) ions which depicted that the complexes were paramagnetic with magnetic moment values ranging from 5.10 to 6.00 BM. Powder XRD studies confirmed that the amino acid complexes were crystalline with monoclinic crystal structure. The in vitro biological activity of the investigated chromium(III) complexes with alanine and valine was tested against Bacillus subtilis, Staphylococcus aureus, Salmonella typhi, Pseudomonas aeruginosa, and Escherichia coli. All the microorganisms were standardized using 0.5 McFarland standard. The antimicrobial studies showed that the ligands were biologically active with an inhibition zone range of 10-17 mm and their metal complexes showed significantly enhanced antimicrobial sensitivity with an inhibition zone range of 12-21 mm. The standard drug showed slightly better activity with an inhibition zone range of 24-38 mm.
Keywords: Synthesis, Characterization, XRD, Biological activity
Copyright and License Information
© 2019 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
In general, bacteria have the genetic ability to cause and acquire resistance to drugs, which are used as therapeutic agents. Although a large number of new antibiotics have been produced in the last three decades, the resistance of microorganisms to these drugs has increased (1). The microbial resistance presents a problem and the use of antimicrobial drugs in the future is still uncertain. Therefore, the measures to reduce or solve this problem include controlling the use of antibiotics, developing the research to better understand the genetic mechanisms of resistance, and continuing researching on how to develop new drugs, either synthetic or natural. The ultimate goal is to offer appropriate and efficient antimicrobial drugs to the patient. For centuries, people have used copper, iron, chromium, cobalt, and other transition metal ions complexes to inhibit the growth of harmful microbes (1). The complexes of transition metals with amino acids have been widely studied for their anti-microbial properties. They have been evaluated against several microorganisms with promising results. In addition to their ability to combat infection or neoplastic disease, these new agents should exhibit selective toxicity, chemical stability, and optimum rates of bio-transformation and elimination (2).
However, amino acids are absorbed well from intestinal lumen by specific active transport mechanisms. They display significant biological activity (3,4) and easily form stable complexes with most transition metal ions (5).The structure of organic compounds has been observed to undergo a significant modification in the addition of metals. In particular, amino acid and their metal complexes have attracted a great deal of attention as anti-cancer, anti-tubercular, anti-convulsant, insecticidal, antibacterial, anti-fungal, anti-biotic, and anti-inflammatory agents (6). The structure of alanine and valine are represented in Figs. 1 and 2, respectively. It is in the light of this challenge that the development of new drugs that are resistant to microorganisms cannot be overemphasized.
Figure 1.
Structure of Alanine.
.
Structure of Alanine.
Figure 2.
Structure of Valine.
.
Structure of Valine.
2. Materials and Methods
2.1. Synthesis and Experimentation
The reagents and solvents were of analytical grade which included Cr(NO3)3.9H2O, ethanol, methanol, Dimethyl formide, Dimethyl sulphoxide, and Amino acids (Valine and Alanine). Clinical bacteria isolates (Bacillus subtilis, Staphylococcus aureus, Salmonella typhi, Pseudomonas aeruginosa,and Escherichia coli) were obtained from Ahmadu Bello University Teaching Hospital, Shika, Zaria, Nigeria. Ciprofloxacin (May and Barker) was used as the positive control.
Additionally, griffin water bath (E-550 model), capillary tube, thermometer (0-360ºC), Fourier-transform infrared spectrophotometer (Cary 630 model, Agilent technologies), UV-visible spectrophotometer (Cary 300 model), melting point apparatus (Gallen Kamp), digital analytical balance (Sartorius ED 2245), magnetic susceptibility balance (Sherwood Scientific, Cambridge, UK) and conductivity meter (Jenway 4020) were used in the study. The X-ray powder diffraction analysis was carried out using Xpert-Pro X-ray diffractometer with Cu Kα1 radiation (λ = 1.54056 Å).
Moreover, funnel, measuring cylinder, water bath, round bottom flask, digital weighing balance, oven, furnace, melting point apparatus, and magnetic stirring hot plate are the equipment and apparatus used for the research.
2.2. Preparation of Ligand Solution
The ligands were prepared by stirring amino acid (BDH, 1.2 g, 2.0 mmol of valine, 1.0 mmol of alanine) with warm distilled water (20 mL, 20 g) in 30% NaOH (BDH, 0.33 mL) until the amino acid dissolved completely (7).
2.3. Preparation of Precursor Solution
The precursor solution was prepared by stirring the mixture of the metal salt (BDH, Cr(NO3)3.9H2O salt) with distilled water (25 mL, 25 g) (7).
2.4. Synthesis of Complexes
The complexes were prepared by stirring the mixtures of precursor solution (20 mL) with amino acid solution (25 mL) at 60ºC for 2 hours. A precipitate was formed, washed with ethanol, recrystallized in methanol and kept in a desiccator (7) as represented in formulas (1) and (2)
CrCl3.9H2O + 3C3H7NO2→[Cr(C3H6NO2)3] + 9H2O + 3HNO3
Cr(III)-Valine Complexation Reaction (1)
CrCl3.9H2O + 3 C5H10NO→[Cr(C5H10NO2)3] + 9H2O + 3HNO3
Cr(III)-Alanine Complexation Reaction (2)
3. Results and Discussion
3.1. Physical Properties of the Synthesized Complexes and the Ligands
The percentage yield of the synthesized complexes ranged between 50 and 70%, the melting points were found to be within the range of 258–298ºC for the ligands and 230–290ºC for the complexes. The complexes were colored and this is due to the fact that most of the transition metal complexes are colored and the color is observed due to d-d transition in the visible region. Analytical data and the properties of the compounds are shown in Table 1.
Table 1.
Analytical Data and Physical Properties of the Ligand and Complexes
|
Complex/Ligand
|
Melting point (
o
C)
|
Colour
|
Conductivity (cm
2
mol
-1
Ω
-1
)
|
% Of Metal Calculated (Observed)
|
| C3H7NO2 (alanine) |
258 |
White |
- |
|
| C5H11NO2 (valine) |
298 |
White |
- |
|
| Cr(III)-alanine |
230-232 |
Purple |
31 |
18.60 (19.20) |
| Cr(III)-Valine |
258-260 |
Purple |
28 |
20.65 ( 21.35) |
3.2. FTIR Results
The characteristic frequencies of the expected functional groups are depicted in Table 2. The assignment of peaks was done based on standard references previously published by several authors. The IR spectra of the ligands (alanine and valine) showed strong absorption bands at 1320 and 1330 cm-1, respectively, which was due to carbon-nitrogen vibration (υ C-N), while in the metal complexes chromium(III)-alanine and chromium(III)-valine, the υ C-N absorption bands were obtained at 1398 and 1167 cm-1, respectively. The new weak intensity bands in the region 541-571 cm-1and 359-489 cm-1 in the spectra of the complexes were assigned to v(M-O) and v(M-N) stretching vibrations, respectively (8-10).
Table 2.
Important IR frequencies of Ligands and Their Metal Complexes (cm -1)
|
Complex/Ligand
|
υ
(N-H)
|
υ
(C-O)
|
υ
(M-O)
|
υ
(M-N)
|
υ
(M-OH)
|
υ
(C-N)
|
| Alanine |
3052 |
1608 |
|
|
|
1320 |
| Valine |
3350 |
1605 |
|
|
|
1330 |
| Cr(III)-AL |
3467 |
1633 |
583 |
431 |
|
1398 |
| Cr(III)-VL |
3416 |
1615 |
537 |
425 |
|
1167 |
The absorption band of the amine group (N-H) ranged between 3448 and 3383 cm-1, suggesting the possibility of the coordination of ligand through the nitrogen atom of the amine group. The N-H stretching vibration at 3119 cm-1 in the ligand was shifted to a higher frequency in the complex, which is an indication that the coordination of the metal ion with the ligand took place via the nitrogen atom (11,12). The absorption frequency band at 1624 cm-1 was ascribed to the C=O stretching vibration in the spectrum of the ligand, and it was shifted to 1578 cm-1and 1584 cm-1 in the complexes, which is an indication of the involvement of this group in metal-ligand bond formation (9). The major peak frequencies are depicted in Table 2.
However, for chromium-alanine complex, the N-H stretching vibration appears at 3416 cm-1, and the lower frequency at 3052 cm-1in the free ligand. This indicates the formation of M-N bond via the amine group. The absorption frequency band at 1608 cm-1 was attributed to C=O stretching in the spectrum of the free ligands and was shifted to a higher frequency at 1615 cm-1 in the chromium-alanine complex. This evidence suggests the formation of M-O bond via the carboxylate group in the ligand. Moreover, the involvement of oxygen and nitrogen in the acid and amino groups in the ligand was further supported by the appearance of band around 432-486 cm-1 for M-O stretching and around 535-700 cm-1 for M-N stretching (4,8).
3.3. UV-Visible Results
The assignments have been done based on standard references and earlier studies published by several authors (13-15). The absorption bands in all the complexes located between 200 and 400 nm were attributed to the organic moiety and the absorption peaks above 400 nm were attributed to the formation of metal-ligand bond. In all the complexes, the n→π* characteristic band assigned to C=O bond appeared at 250–370 nm, while it appeared at 270–290 nm in the ligands spectrum, which also supported the involvement of carboxylate ion in the complex formation. The bands attributed to π→π* transitions in the complexes appeared at 270-390 nm, whereas in the free ligands, they were found at 270–280 nm. The presence of absorption band within 269-378 nm in the complexes was due to n→σ* transition that was found at 269- 278 nm in the free ligands. The presence of π→π*, n→π*, and n→σ* band in all the complexes indicated the presence of the functional groups of the parent ligands in the complex (C=O and NH2).
A large shifting of the absorption band in all the complexes caused the appearance of a new band for d-d electronic transition, which indicates the possibility of forming metal-ligand coordination bond in the complexes. Transition complexes are generally colored and their colors are observed due to the absorption of light in the visible region. Therefore, the bands appearing at above 400 nm in all the complexes were clearly observed due to d-d electronic transitions that occurred in the complexes (15) as seen in Table 3.
Table 3.
Electronic Transition (nm) of the Ligands and Complexes
|
Complex/Ligands
|
π→π*
|
n→σ*
|
π→π*
|
d-d
|
| Alanine |
250 |
269 |
270 |
|
| Valine |
260 |
278 |
280 |
|
| Cr(III)-alanine |
333 |
347 |
298 |
566 |
| Cr(III)-valine |
345 |
389 |
368 |
546 |
3.4. Magnetic Susceptibility
The complexes of Cr(III) are high spin paramagnetic compounds as suggested by their magnetic moment values (16-20). The values of the magnetic moments of these complexes were in the range of 4.3-5.2 as represented in Table 4, which are comparable with the values reported for octahedral chromium(III) complexes (17-19). The value of the magnetic moments of these types of complexes was favorable for the formation of an octahedral complex. Therefore, the proposed geometry for all these complexes was octahedral.
Table 4.
Result of Magnetic Measurement of the Complexes
|
Complex
|
Χ
× 10
-5
(cgs)
|
Χ
m
× 10
-2
(cgs)
|
µ
eff
Calculated (observed) (BM)
|
| Cr(III)-AL |
1.60 |
6.45 |
3.87(3.95) |
| Cr(III)-VL |
1.86 |
5.90 |
3.87(3.78) |
3.5. Molar Conductivity
The molar conductivity values of Cr-alanine and Cr-valine were 25 and 30 cm2 Ω-1 mol-1, respectively, as shown in Table 5. This value showed that these complexes are not electrolytic in nature (20).
Table 5.
Molar Conductivities Values of the Synthesized Complexes
|
Compounds/Ligands
|
Molar Conductivity ˄ (cm
2
Ω
-1
mol
-1
)
|
| C3H7NO2 ( alanine) |
|
| C5H11NO2 (valine) |
|
| [Cr(C3H6NO2)3] |
25 |
| [Cr(C5H10NO2)3] |
30 |
3.6. X-Ray Diffraction Results
The diffractogram of Cr(III) complex of valine had ten reflections with maxima at 2θ = 14.10° corresponding to a d value of 2.384 Å. However, the diffractogram of Cr(III) complex of alanine had only three reflections with maxima at 2θ = 10.20° corresponding to a d value of 2.60 Å as depicted in Figs. 3 and 4, respectively. However, a summary of the crystal information is presented in Table 6 and the spectra are represented in Figs. 3 and 4, respectively.
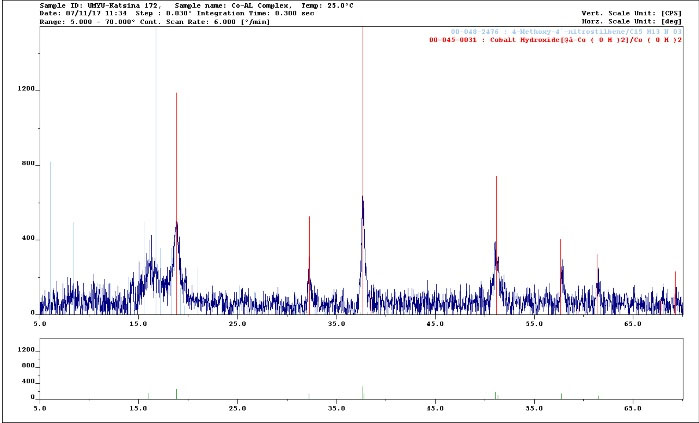
Figure 3.
XRD Spectrum of Cr(III)-valine Complex (λ Cu Kα = 1.54056 Å).
.
XRD Spectrum of Cr(III)-valine Complex (λ Cu Kα = 1.54056 Å).
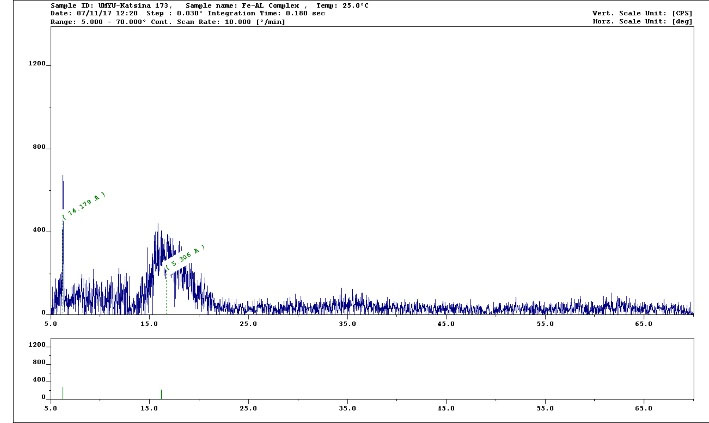
Figure 4.
XRD Spectrum of Cr(III)-Alanine Complex (λ Cu Kα = 1.54056 Å).
.
XRD Spectrum of Cr(III)-Alanine Complex (λ Cu Kα = 1.54056 Å).
Table 6.
Summary of Crystal Data of the Complexes
|
|
Cr- AL
|
Cr-VL
|
| a, b, c (Ȃ) |
6.37, 10.30, 15.25 |
5.12, 8.89, 12.0 |
| α, β, γ |
90, 90, 78 |
90, 90, 120 |
| Size (Ȃ) |
132 |
156 |
| System |
Monoclinic |
Monoclinic |
| Geometry |
Octahedral |
Octahedral |
3.7. Metal Content Analysis
The percentage composition of the metal ions in the complexes ranged from 12.70 to 21.35 %, as presented in Table 7.
Table 7.
Metal Content Analysis of the Complexes
|
Complexes
|
Weight of Complex (g)
|
Weight of Oxide (g)
|
Gravimetric Factor
|
% of metal Calculated
|
% of Metal Observed
|
| Cr(III)-AL |
0.10 |
0.0222 |
0.8725 |
18.60 |
19.20 |
| Cr(III)-VL |
0.10 |
0.0245 |
0.8725 |
20.65 |
21.35 |
Note: AL=Alanine, VL=Valine
3.8. Water Content Analysis
The percentages of the water content of Cr-alanine and Cr-valine were 4.2% and 5.20%, respectively, as shown in Table 8.
Table 8.
Water Content Analysis of the Complexes
|
Complexes
|
Initial Weight (g)
|
Final Weight (g)
|
Loss in Weight (g)
|
% Of Water Calculated
|
% Of Water Observed
|
| Cr(III)-AL |
0.20 |
0.1958 |
0.0042 |
5.40 |
4.20 |
| Cr(III)-VL |
0.20 |
0.1948 |
0.0052 |
7.10 |
5.20 |
Note: AL=alanine, VL=valine
3.9. Biological Activity
The results of the inhibitory activity (sensitivity test) showing the inhibition zones (mm) were obtained within the range of 8–14, 8–10, 11–18 and 13–39 mm for the ligands, complexes, and standard drugs respectively as presented in Table 9. Moreover, Tables 10 and 11 show the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC), respectively. In which, the concentration of the compounds (ligands, complexes and standard drugs) was found to be within the range of 12.5–25 mg/mL for the MIC and 25–50 mg/mL for the MBC.
Table 9.
The Diameter of Zone of Inhibition (mm) at Varying Concentration (mg/mL) of the Complexes and Ciprofloxacin
|
|
Cr(III)-VL Complex
|
Cr(III)-AL Complex
|
Ciprofloxacin
|
| Test organism/ concentration (mg/mL) |
100 |
50 |
25 |
12.5 |
100 |
50 |
25 |
12.5 |
|
|
S. aureus
|
22 |
17 |
15 |
12 |
18 |
16 |
13 |
10 |
35 |
|
B. Subtilis
|
21 |
18 |
16 |
14 |
21 |
17 |
14 |
12 |
32 |
|
E. coli
|
19 |
16 |
14 |
- |
16 |
13 |
10 |
- |
37 |
|
S. typhi
|
18 |
15 |
13 |
- |
17 |
15 |
- |
- |
28 |
|
P. aeruginosa
|
16 |
13 |
- |
- |
18 |
15 |
- |
- |
36 |
Table 10.
Minimum Inhibitory Concentration (MIC) of the Complexes (mg/L)
|
Test Organism
|
Cr(III)-VL
|
Cr(III)-AL
|
|
S. aureus
|
12.5 |
25 |
|
B. Subtilis
|
12.5 |
25 |
|
E. coli
|
25 |
25 |
|
S. typhi
|
25 |
50 |
|
P. aeruginosa
|
50 |
50 |
Note: AL, alanine, VL, valine.
Table 11.
Minimum Bactericidal Concentration (MBC) of the Complexes (mg/L)
| Test organism |
Cr(III)-VL |
Cr(III)-AL |
|
S. aureus
|
25 |
25 |
|
B. Subtilis
|
25 |
50 |
|
E. coli
|
50 |
50 |
|
S. typhi
|
50 |
100 |
|
P. aeruginosa
|
100 |
100 |
Note: AL, alanine, VL, valine
A comparative evaluation of the antibacterial activity of the complexes and ciprofloxacin was carried out against five test organisms. The result indicated that both complexes and ciprofloxacin were active against all the test organisms.All other complexes showed potency against the pathogen and were more active than the parent ligand, suggesting the enhanced lipophilicity of the complexes on coordination as reported in previously published works (21-23). However, the organism appeared to be susceptible to Cr-alanine with greater activity shown by the complex than the ligand as reported in the literature (23,24), and the standard drug (ciprofloxacin) showed better activity against all the Gram-negative bacteria. Therefore, this outcome suggests the engagement of these complexes in the development of new antibacterial agents for therapeutic applications. All the complexes showed potency against Staphylococcus aureus. This outcome is fascinating since S. aureus causes food poisoning and is highly resistant to most antibiotics.
Generally, the Gram-positive bacteria proved to be more susceptible to the amino acid complexes than the Gram-negative bacteria. The weak antibacterial activity against gram-negative bacteria may be ascribed to the presence of an outer membrane which poses hydrophilic polysaccharide chains as a barrier to the amino acid complexes (23,24). Therefore, the comparative studies of the ciprofloxacin and metal complexes indicated that the complexes showed antimicrobial activity against the studied microbial strains as reported by Kabbani et al (24).
Additionally, from the above analyses, the proposed structures for these complexes are shown in Figs. 5 and 6.
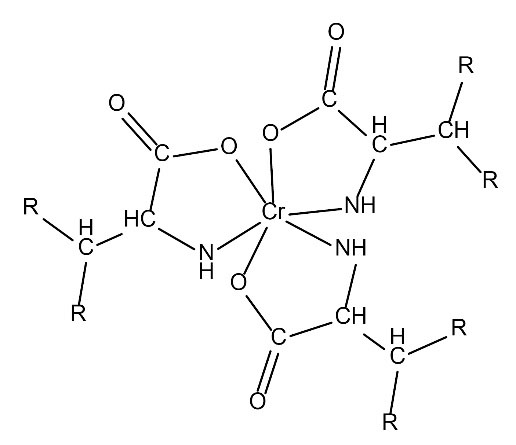
Figure 5.
Proposed Structure of Cr(III)–Valine.
.
Proposed Structure of Cr(III)–Valine.
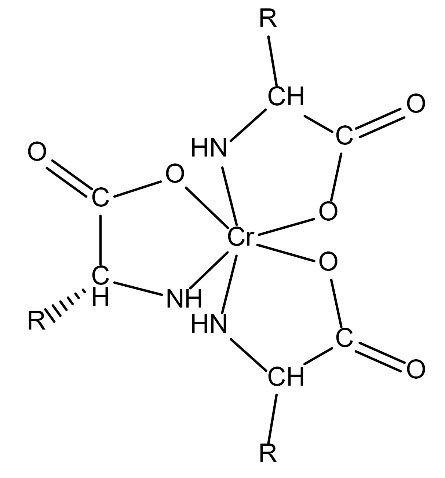
Figure 6.
Proposed Structureof Cr(III)–alanine (R=CH3-).
.
Proposed Structureof Cr(III)–alanine (R=CH3-).
4. Conclusion
The complexes of Cr(III) with alanine and valine have been synthesized in basic aqueous medium and characterized. The molar conductivity measurements showed a ratio of 1:3. The IR spectra showed that the amino acids acted as bidentate ligands with coordination involving the carboxyl oxygen and the nitrogen of the amino group. Magnetic susceptibility measurements suggested a six-coordinate local symmetry around the complexes. Additionally, magnetic susceptibility studies revealed that all the complexes were paramagnetic. Metal content and water content analyses showed that the complexes contained no water of crystallization. Powder XRD studies confirmed that the amino acid complexes were crystalline in nature and that they largely crystallized in monoclinic fashion. Moreover, it suggested that the bonding mode in the complexes was similar. In general, the complexes can be represented by the formula [M1L3] (where M1=Cr(III), L=Valine anion, alanine anion). The complexes were stable in the air and soluble in DMF and DMSO. The low molar conductance values (10-3 M) of solutions in DMSO indicated that all the complexes behaved as non-electrolytes. The antimicrobial studies suggested that the amino acid ligands were biologically active and their metal complexes showed significantly enhanced antimicrobial sensitivity against the studied microbial strains in comparison to the free ligands
Conflict of Interests
There is no conflict of interest.
Author’s Contribution
The authors were all supportive and help in the research.
References
- Raman N, Kulandaisamy A, Jeyasubramanian K. Synthesis, spectroscopic characterization, redox, and biological screening studies of some Schiff base transition metal (II) complexes derived from salicylidene-4-aminoantipyrine and 2-aminophenol/2-aminothiophenol. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry 2001; 31(7):1249-70. doi: 10.1081/SIM-100106862 [Crossref] [ Google Scholar]
- Johari R, Kumar G, Kumar D, Singh S. Synthesis and antibacterial activity of M(II) schiff-base complex. J Indian Council Chem 2009; 26:23-7. [ Google Scholar]
- Lekha L, Raja KK, Rajagopal G, Easwaramoorthy D. Synthesis, spectroscopic characterization and antibacterial studies of lanthanide(III) Schiff base complexes containing N, O donor atoms. J Mol Struct 2014; 1056-1057:307-13. doi: 10.1016/j.molstruc.2013.10.014 [Crossref] [ Google Scholar]
- Devi P. P, Lonibala R. Synthesis, Characterization and Antimicrobial Studies of Mn(II), Co(II), Ni(II) Cu(II) and Zn(II) Complexes of (R,E)-2-(Dimethylamino)-3-(4-Hydroxyphenyl)-N,2,3-Trimethyl-N'-(1-P-Tolylethylidene) Butanehydrazide. Orient J Chem 2017;33(3). 10.13005/ojc/330331 .
- Kulkarini AD, Patil SA, Badami PS. Electrochemical properties of some transition metal complexes: synthesis, characterization and in-vitro antimicrobial studies of Co(II), Ni(II), Cu(II), Mn(II) and Fe(III) complexes. Int J Electrochem Sci 2009; 4(5):717-29. [ Google Scholar]
- Stănilă A, Braicu C, Stănilă S, Pop RM. Antibacterial activity of copper and cobalt amino acids complexes. Not Bot Horti Agrobot Cluj Napoca 2011; 39(2):124-9. doi: 10.15835/nbha3926847 [Crossref] [ Google Scholar]
- Nelson DL, Cox MM. Lehninger Principles of Biochemistry. 3rd ed. New York: Macmillan Worth Publishers; 2011.
- Nakamoto K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. 3rd ed. New York: John Wiley and Sons; 1988. p. 211.
- Joseyphus RS, Dhanaraj CJ, Nair MS. Synthesis and characterization of some Schiff base transition metal complexes derived from vanillin and L(+)alanine. Transit Met Chem 2006; 31(6):699-702. doi: 10.1007/s11243-006-0048-7 [Crossref] [ Google Scholar]
- Stanila A, Marcu A, Rusu D, Rusu M, David L. Spectroscopic studies of some copper(II) complexes with amino acids. J Mol Struct 2007; 834-836:364-8. doi: 10.1016/j.molstruc.2006.11.048 [Crossref] [ Google Scholar]
- Solanki MR, Acharya GD, Hathi MV. Synthesis characterization and biological investigations on metal Complexes of 2-[(8-Hydroxy-1-quinolin-5-yl) methyl]-1H-isoindole-1, 3 (2H) dione. E- J Chem 2009; 6(4):1023-8. doi: 10.1155/2009/879048 [Crossref] [ Google Scholar]
- Bhagat TM, Swamy Swamy, DK DK, Deshpande MN. Synthesis and characterization of transition metal complexes with newly synthesized substituted benzothiazole. J Chem Pharm Res 2012; 4(1):100-4. [ Google Scholar]
- Stanila A, Nagy C, Marcu A, Cozma D, Rusu D, David L. Spectroscopic investigations of new metallic complexes with leucine as ligand. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 2009; 267(2):419-21. doi: 10.1016/j.nimb.2008.10.024 [Crossref] [ Google Scholar]
- Liang Y, Su B, Zhao J, Sun W. the synthesis of new asymmetric double Schiff bases containing a new o‐amino benzoic acid derivative. Synth Commun 2004; 34(17):3235-42. doi: 10.1081/SCC-200028639 [Crossref] [ Google Scholar]
- El-Megharbel SM, El-Sayed MY. Synthesis and thermal studies of MnII, CrIII and FeIII methionine complexes. Life Sci J 2012; 9(2):1254-9. [ Google Scholar]
- Earnshaw A. Introduction of Magnetochemistry. London: Academic Press; 1968. p. 23-30.
- Munde AS, Jagdale AN, Jadhav SM, Chondhekar TK. Synthesis, characterization and thermal study of some transition metal complexes of an asymmetrical tetradentate Schiff base ligand. J Serb Chem Soc 2010; 75(3):349-59. doi: 10.2298/jsc090408009m [Crossref] [ Google Scholar]
- El-Behery M, El-Twigry H. Synthesis, magnetic, spectral, and antimicrobial studies of Cu(II), Ni(II) Co(II), Fe(III), and UO2(II) complexes of a new Schiff base hydrazone derived from 7-chloro-4-hydrazinoquinoline. Spectrochim Acta A Mol Biomol Spectrosc 2007; 66(1):28-36. doi: 10.1016/j.saa.2006.02.017 [Crossref] [ Google Scholar]
- Nabeel BH, Farah ST. Synthesis and structural studies on some transition metal complexes of Bis-(benzimidazole-2-thio) ethane, propane and butane ligands. Res J Chem Sci 2012; 2(6):43-9. [ Google Scholar]
- Geary WJ. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev 1971; 7(1):81-122. doi: 10.1016/S0010-8545(00)80009-0 [Crossref] [ Google Scholar]
- Sadlers P, Muncia C, Shipman MA. Metals in medicine. In: Bertini I, Gray HB, Stiefel EI, Valentine JS, eds. Biological Inorganic Chemistry: Structure and Reactivity. London, UK: University Science Books; 2007. p. 115-36.
- Cao WX, Cheng QM, Fei XF, Li SF, Yin HR, Lin YZ. A study of preoperative methionine-depleting parenteral nutrition plus chemotherapy in gastric cancer patients. World J Gastroenterol 2000; 6(2):255-8. doi: 10.3748/wjg.v6.i2.255 [Crossref] [ Google Scholar]
- Walsh CT, Fisher SL, Park IS, Prahalad M, Wu Z. Bacterial resistance to vancomycin: five genes and one missing hydrogen bond tell the story. Chem Biol 1996; 3(1):21-8. doi: 10.1016/s1074-5521(96)90079-4 [Crossref] [ Google Scholar]
- Kabbani AT, Hammud HH, Ghannoum AM. Preparation and antibacterial activity of copper and cobalt complexes of 4-chloro-3-nitrobenzoate with a nitrogen donor ligand. Chem Pharm Bull (Tokyo) 2007; 55(3):446-50. doi: 10.1248/cpb.55.446 [Crossref] [ Google Scholar]