Avicenna J Environ Health Eng. 6(2):66-74.
doi: 10.34172/ajehe.2019.09
Original Article
Adsorption of Nickel and Chromium From Aqueous Solutions Using Copper Oxide Nanoparticles: Adsorption Isotherms, Kinetic Modeling, and Thermodynamic Studies
Razieh Hosseini  , Mohammad Hossein Sayadi *
, Mohammad Hossein Sayadi *  , Hossein Shekari
, Hossein Shekari
Author information:
Department of Environmental Engineering, Faculty of natural resources and Environment, University of Birjand, Birjand, Iran.
*Correspondence to Mohammad Hossein Sayadi, Tel.: +98 5632254068 Fax: +98 5632254066 Email:
mh_sayadi@birjand.ac.ir
Abstract
The research was conducted with an aim to assess the efficiency of copper oxide nanoparticles as an adsorbent to remove Ni and Cr. The effect of pH, adsorbent dosage, contact time, initial concentration of metals (Ni and Cr) on the adsorption rate was evaluated and removal of these elements from aqueous solutions was measured using Atomic Absorption Spectrum System (Conter AA700). Moreover, the kinetic and isotherm besides thermodynamic adsorption models were assessed. The highest Ni and Cr removal rate occurred at an optimal pH of 7, and an initial concentration of 30 mg/L, a time period of 30 minutes, and 1 g/L of copper oxide nanoparticles. In fact, with the increase of adsorbent dosage and contact time, the removal efficiency increased and with initial concentration increase of Ni and Cr ions, the removal efficiency reduced. The correlation coefficient of isotherm models viz. Langmuir, Freundlich, Temkin, Redlich-Peterson, and Koble-Corrigan showed that Ni and Cr adsorption via copper oxide nanoparticles better follows the Langmuir model in relation to other models. The results showed that kinetic adsorption of Ni and Cr via copper oxide nanoparticles follows the second order pseudo model with correlation coefficients above 0.99. In addition, the achieved thermodynamic constants revealed that the adsorption process of metals (i.e., Ni and Cr) via copper oxide nanoparticles was endothermic and spontaneous and the reaction enthalpy values for these metals were 17.727 and 11.862 kJ/mol, respectively. In conclusion, copper oxide nanoparticles can be used as effective and environmentally compatible adsorbents to remove Ni and Cr ions from the aqueous solutions.
Keywords: Ni, Cr, Thermodynamics, Adsorption
Copyright and License Information
© 2019 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
The access to safe, reliable and non-contaminated drinking water resources is the prime need of mankind to have a dynamic and stable society. The urbanization development and expansion of agricultural and industrial activities besides indiscriminate use of resources have led to the expansion of environmental pollution. The high toxicity of heavy metals has caused serious issues at low concentrations for natural ecosystems (1). Heavy metals are reported to be involved in neurological disorders (Parkinson, Alzheimer, depression, and schizophrenia), varied cancer types, nutrient deficiency, disruption of hormone balance, respiratory and cardiovascular disorders, hair loss, osteoporosis, damage to the liver, kidneys and brain besides abortion, anorexia, joint inflammation and in severe cases death in human beings (2). In fact, the entry of harmful materials into the environmental cycle threatens the viability of life on Earth; therefore, identification and elimination of toxic contaminants are essential for the protection of an environment (3). Contamination of water resources viacontaminants is a rising issue worldwide; therefore, in the context of pollution control, the removal of these heavy metals from aqueous solutions is of special importance (4). Among the most important nano-technology applications in an environment, its use to reduce the emission of contaminants, filter water and wastewater, eliminate pollutants and environment monitor can be addressed (5). Considering the dangers arising from heavy metals, it is imperative that in line with environmental standards and regulations, these contaminants are eliminated before draining and entering an environment. This context has always been considered by the environmental researchers and specialists; therefore, for this purpose, several researches have been carried out for application and use of the methods that are simply applicable, cost-effective and environment-friendly. Different methods exist to eliminate the heavy metals from aqueous environments which include coagulation, oxidation, chemical sequestration, ion-exchange, reverse osmosis, and electrodialysis; these methods are time-consuming and costly (6). However, it can be said that an alternative method is adsorption that in addition to simplicity and cost-effectiveness has a high efficiency. The adsorption via metal nanoparticles is a technology compatible with the environment that can be used as an effective method to eliminate heavy metals and organic contaminants from water and wastewater (7). The isotherm is the most important parameter in the design of adsorption systems and describes the relationship between the concentration of adsorbent and the adsorption capacity of an adsorbent; in fact, isotherms are the basis for describing the adsorbent and adsorbent behaviors as well as presenting the most important type of adsorption type (8). In nanometre scales, the particles with unique electronic, magnetic, optic and catalytic properties are adsorbed. The vast application of nanoparticles in adsorption can be considered due to high adsorption efficiency, high reactivity, more active sites and the ability of nanoparticles to disperse in the aqueous solutions. In addition, the nanoparticles due to their tiny size and high surface to mass ratio provide a suitable condition to adsorb metal ions from aqueous solutions (9). The results of a study by Shekari et al showed that FeNi3/TiO2 magnetic nanocomposite can remove 82.67% of hexavalent chromium with an initial concentration of 0.5 mg/L (10). Therefore, the aim of this research was to use copper oxide nanoparticles as an adsorbent to remove Ni and Cr from aqueous solutions.
2. Materials and Methods
In this study, copper oxide (CuO) nanoparticles with 99% purity produced by US Research Nanomaterials Company (Houston, TX 77084, USA) and Sodium hydroxide (NaOH), Nitric acid (HNO3) with 65% purity from Merck (German) have been used. Moreover, pH-meter (Istek, 915PDC, Korea), digital scale (Kern, ABJ220-4M, Japan), shaker (Eyela, MMS-210, Japan), and Atomic Adsorption Spectrometry (Analytikjena, ContrAA700, Germany) were used.
2.1. Adsorption Experiments
Initially, 50 mL of Ni and Cr ions with specified and equal concentrations was prepared from nickel chloride and potassium dichromate salts for the conduction of the adsorption process using copper oxide nanoparticles. The parameters including pH, adsorbent dosage, contact time and initial concentration of Ni and Cr were assessed. The solution pH was evaluated and adjusted to the range of 2-8 (2,3,4,5,7,8), with 0.1M NaOH and HNO3 solutions. To determine the effect of adsorbent dosage on Ni and Cr adsorption, different adsorbent values in the range of 0.25-2 g/L were studied. The equilibrium time and adsorption kinetics were determined at the time span of 5-120 minutes. The effect of the initial concentration of Ni and Cr on their adsorption rate at the range of 10-50 mg/L was evaluated at an optimal pH, adsorption dosage and contact time of the previous stage (9,10). After the adsorption experiments were carried out for a specified time, the samples were centrifuged to separate the copper oxide nanoparticles. Finally, the analysis of samples containing heavy metals (Ni and Cr) was carried out using the Atomic Adsorption System (Contr AA700).
To calculate Ni and Cr removal efficiency, the (Equation 1) was used
Besides, the adsorbent equilibrium capacity was calculated using the equation (equation 2).
Where qe is the quality of adsorbed metal ions per adsorbent mass unit, Co is initial concentration of metal ions present in the solution mg/L, Ce is the equilibrium concentration of metal ions in the solution mg/L, V is the solution volume in L, and m is adsorbent weight in g (11).
2.2. Adsorption Isotherms
To assess the performance of adsorbent, different equations and isotherms exist, out of which the Langmuir, Freundlich, Temkin, Redlich-Peterson and Koble-Corrigan isotherms were used that have been presented in Table 1. Besides, the isotherm models were applied at optimal conditions of the parameters.
Table 1.
Adsorption Isotherm Models
|
Isotherms
|
Equations
|
Linear expression
|
Plot
|
Parameters
|
| Langmuir (12) |
|
|
|
qm =(slope)-1
KL=slope/intercept |
| Freundlich (13) |
|
|
|
KF =exp(intercept)
n=(slope)-1 |
| Temkin (14) |
|
|
|
KT =exp (intercept/slope)
BT=slope |
| Redlich-Peterson (15) |
|
|
|
g=slope
BRP =exp (intercept)
ARP* |
| Koble-Corrigan (16) |
|
|
|
AKC = (slope)-1
BKC = intercept/slope
P* |
2.3. Kinetic Adsorption
To study the kinetic adsorption of Ni and Cr by CuO nanoparticles, the adsorption experiments with an initial concentration of 30 mg/L of Ni and Cr, 1 g/L of an adsorbent, pH=7 at a speed of 150 rpm in the shaker at contact times of 5, 15, 30. 60. 90 and 120 minutes respectively were carried out. The kinetic data were analyzed using some of the known kinetic models that have been stated in Table 2.
Table 2.
Kinetic Models and Their Linearized Expressions
|
Kinetic Model
|
Equations
|
Linear Expression
|
Plot
|
Parameters
|
| Pseudo-first order (17) |
𝑞𝑡=𝑞e(1- exp(-𝑘1P 𝑡)) |
𝐿og(𝑞𝑒-𝑞𝑡)=Logqe (𝑘1/2.303)𝑡 |
Log( q
e
-q
t
) vs. t
|
q
e=exp(intercept)
k1=-(slope) |
| Pseudo-second order (18) |
𝑞t=k2P𝑞e2t/(1+qek2P) |
t/q
t =1/k2Pqe2+1/qe |
t/q
t vs. t |
q
e= (slope)-1
k2=(slope)2/intercept |
2.4. Studies of Thermodynamic Adsorption Process
The results of thermodynamic studies can provide valuable information about the adsorption process and preparations for higher adsorption efficiency. The thermodynamic parameters included variations of the Gibbs free energy (ΔGº) besides Enthalpy (ΔHº) and Entropy (ΔHº) which are the most important specifications of an adsorption process in the scientific applications are calculated using equations 3-5.
Where R is the universal gas constant equivalent to 8314.3 J/mol.K, qe is metal dosage adsorbed on the unit of adsorbent mass in mg/g, Ce is the equilibrium concentration of metal ions in the solution in mg/L and T is the temperature in Kelvin degrees (19).
The average enthalpy changes ΔHº are calculated using Van’t Hoff equation:
(5)
To assess thermodynamic adsorption, the experiments were carried out at an optimal condition of pH=7, adsorbent dosage of 1 g/L, initial concentration of 30 mg/L Ni and Cr, at a speed of 150 rpm in a shaker at 25, 30, 40, 50, and 60ºC, respectively, during a period of 30 minutes.
3. Results and Discussion
3.1. Characteristics of CuO Nanoparticles
CuO nanoparticles have a purity of 99% with particle size of 40 nm, specific surface area (SSA) of 20 m2/g, bulk density of 0.79 g/cm3, true density of 6.4 g/m3, black color and nearly spherical morphology.
3.2. Assessing the Effect of Solution pH on Ni and Cr Adsorption Rate
Fig. 1 illustrates the effect of pH on Ni and Cr removal efficiency via copper oxide nanoparticles. As it is observed, the maximum Cr and Ni adsorption rates were observed at pH 3 and 8, respectively. However, since Ni shows chemical deposition at pH>9, the pH values over 8 were not subjected to experimentation. In general, it can be said that Ni and Cr adsorption were approximately equivalent at pH 7. Considering this, the adsorption rate of metals was compared without consideration of optimal pH for each of them, and the other experiments were conducted at pH 7.
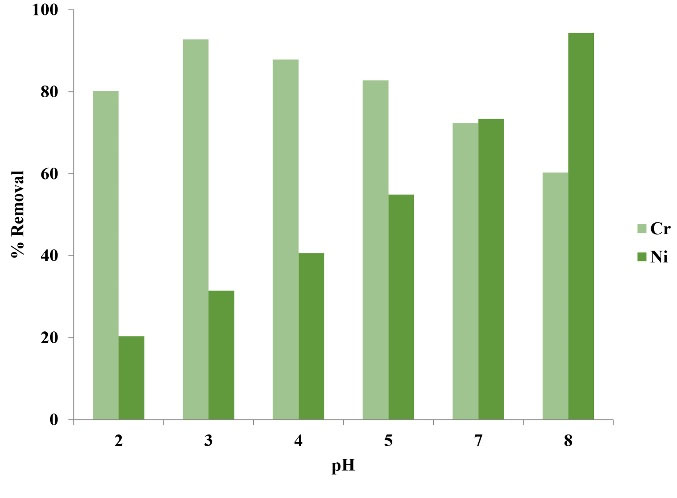
Figure 1.
The Effect of Solution pH on Adsorption Rate of Ni and Cr Ions.
.
The Effect of Solution pH on Adsorption Rate of Ni and Cr Ions.
The pH is one of the most effective parameters in the adsorption process; therefore, its adjustment has a considerable effect on the removal rate of heavy metals in the aqueous environments. At acidic pH values, the protons present on the adsorbent surface easily separate and the adsorbent surface gains negative charge; therefore, the adsorption rate of Cr increases due to the formation of electrostatic attraction force (20). With the increase of pH, the rate of Cr adsorption reduces viaadsorbent since OH- ions concentration in the solution increases and this causes competition between these ions and Cr ions to get adsorbed on the active sites of the surface of the adsorbent (21).
In alkaline solution, sedimentation plays a phenomenal role in the removal of Ni ions; likewise, the sedimentation probability of metal hydroxides in the cavities and in the spaces surrounding the particles is very low since the adsorption process is faster than the sedimentation process from the kinetic viewpoint (22). Therefore, it can be said that Ni removal at pH values lower than 9 is predominantly controlled via adsorption process and at pH values higher than 9, it can be expected that this value increases with Ni hydroxide sedimentation. The results of a study by Wang et al showed that Cr adsorption rate increases with the reduction of pH (23). Furthermore, the results of a study by Xin et al on nickel removal are in concurrence with the results of the present study (24).
3.3. Assessing the Effect of Contact Time on Ni and Cr Adsorption Efficiency
As observed in Fig. 2, adsorption speed in the first minutes was high and with the lapse of time, the adsorption efficiency was almost constant. The highest Ni and Cr adsorption rate was achieved with the lapse of time. This was due to an increase in interaction between metal ions and active sites of the adsorbent surface. The experiments were continued for half an hour and the results showed that with the increase of contact time, the adsorption rate did not indicate a considerable change, which was due to the saturation of adsorbent surface.
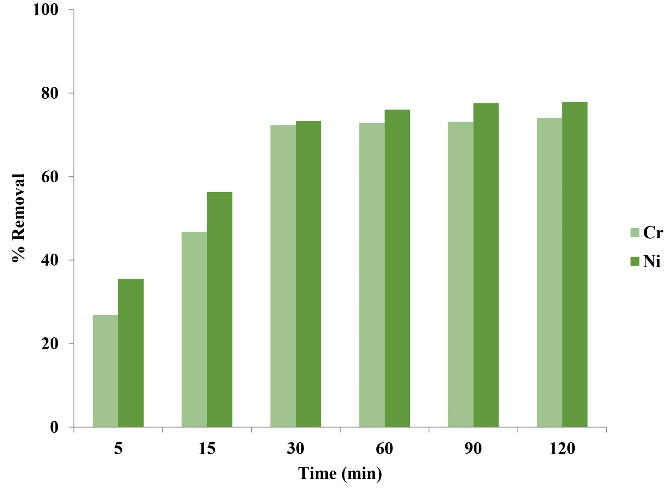
Figure 2.
The Effect of Contact Time on Adsorption Rate of Ni and Cr.
.
The Effect of Contact Time on Adsorption Rate of Ni and Cr.
As observed in Fig. 3, the removal efficiency reached its highest level in the initial 30 minutes, showing the high speed and ability of copper oxide nanoparticles to remove Ni and Cr ions. In fact, as contact time increases, the possibility of higher contact of ions with functional groups existing on the adsorbent surface (adsorbent sites) is provided and adsorption rate increases, but these empty spaces are occupied by Ni and Cr with the lapse of time, leading to the reduction in adsorption capacity (25). The reason for constant adsorption rate is its relationship with the adsorption capacity since with saturation of adsorbent surface, the adsorption rate of Ni and Cr reduces; therefore, it can be said that the two phases (solid and liquid) have reached an equilibrium state (26). The findings of a study by Altun and Pehlivan on chromium removal are in concurrence with the results of the present study (27). Moreover, Pashai Gatabi et al achieved results similar to the results of this stage of the present study using the tiny magnetic multiwalled carbon nanotube nanoadsorbents. They reported that the reaction was initially fast and reached an equilibrium state with the lapse of time (28).
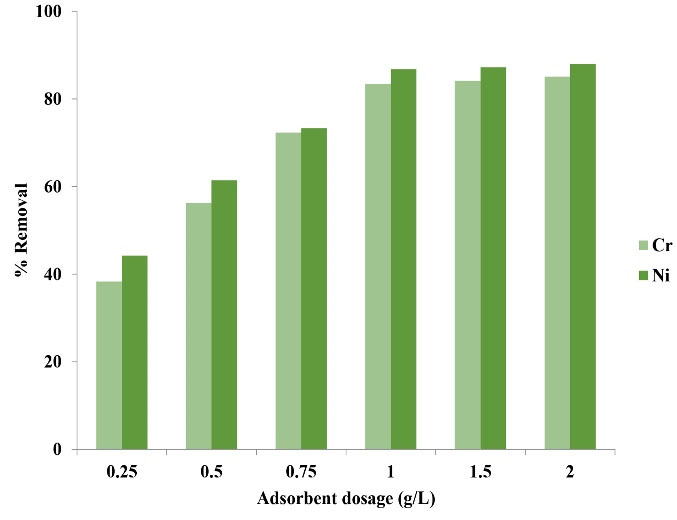
Figure 3.
The Effect of Adsorbent Dosage on Removal Efficiency of Ni and Cr Ions.
.
The Effect of Adsorbent Dosage on Removal Efficiency of Ni and Cr Ions.
3.4. Assessing the Effect of Adsorbent Dosage on the Removal Efficiency of Ni and Cr ions
The results of the effect of adsorbent dosage (copper oxide nanoparticles) on adsorption rate of metals (Ni and Cr) are depicted in Fig. 3. As it is observed in the figure, with the increase of adsorbent dosage, Ni and Cr adsorption rate increases. The number of accessible adsorption sites and the adsorption rate of the metals used in this study increase with increase of adsorbent dosage; however, this trend continued up to the specified adsorbent dosage against a specified concentration of Ni and Cr and after that a considerable change was not observed in the adsorption rate with the increase of adsorbent dosage (copper oxide nanoparticles).
In this research, with the increase of adsorbent dosage, Ni and Cr removal rate increased, and the increasing trend of removal of these metals was due to increase of the number of active adsorption sites or increase of the surface of special adsorbent. In fact, with the increase of adsorbent dosage, the efficiency of adsorption process increased, but adsorption capacity reduced which was due to non-saturation of sites in the adsorptive reactions (29). The results of this stage in the present study are in concurrence with the results of a study by Hong et al (30). Furthermore, Feng et al, who used hydroxyapatite magnetic particles in their study, reported that the removal efficiency increases with the increase of adsorbent dosage (31).
3.5. The Effect of Initial Concentration of Ni and Cr on Their Adsorption Rate
The results of Fig. 4 revealed that with the increase of Ni and Cr ions concentration, the removal efficiency reduced in a manner that it reached its lowest rate at 50 mg/L concentration. This reduction was due to surface constant of copper oxide nanoparticles. Therefore, the increase of Ni and Cr concentration leads to the reduction of adsorption rate and removal efficiency.
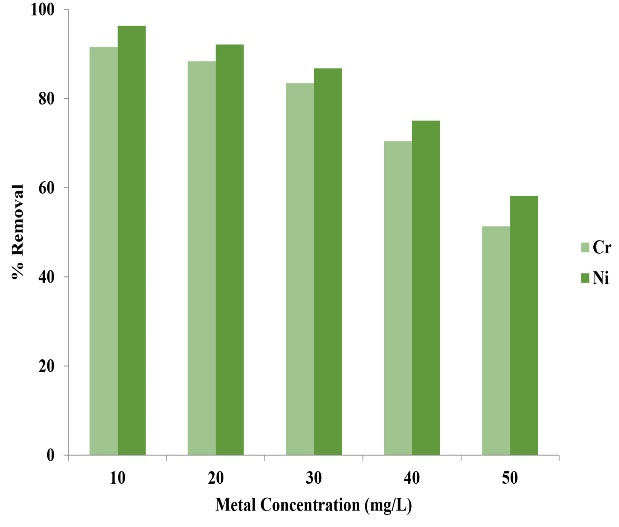
Figure 4.
Effect of Initial Concentration of Ni and Cr on their Adsorption Rate.
.
Effect of Initial Concentration of Ni and Cr on their Adsorption Rate.
With the increase of initial concentration of Ni and Cr ions, their adsorption rate reduces. In fact, the results stated that the removal efficiency of Ni and Cr has an inverse relationship with its initial concentration. This finding signifies that adsorbents have a limited number of active sites that are saturated at high concentration since with the increase of the initial concentration of metal ions, the number of ions competing for reaction with the functional groups on the surface of adsorbent increases and the adsorption sites are saturated; likewise, the increase of metal ions concentration leads to an increase in the interaction between these ions and the adsorbent, speeding up the adsorption process (32). The results of a study by Gupta and Babu on the removal of hexavalent chromium using sawdust are in concurrence with the results of the present study (33). A study conducted by Mahdavi et al on the removal of heavy metals showed that with the increase of metal ions concentration, the removal efficiency reduces, which is in concurrence with the results of the present study (34).
3.6. Determination of Adsorption Isotherm Model
Different experiments were carried out to assess adsorption isotherms based on basic pH conditions, contact time and optimal adsorbent rate with varied initial concentrations of Ni and Cr. The isotherm curves were constructed using the results of adsorption experiment (Figs. 5 to 9). Constant and correlation coefficients of the adsorption isotherm models are depicted in Table 3.
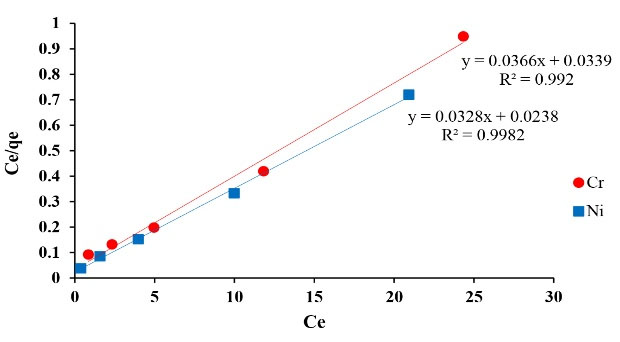
Figure 5.
The Plot of Langmuir Isotherm of Ni and Cr Adsorption.
.
The Plot of Langmuir Isotherm of Ni and Cr Adsorption.
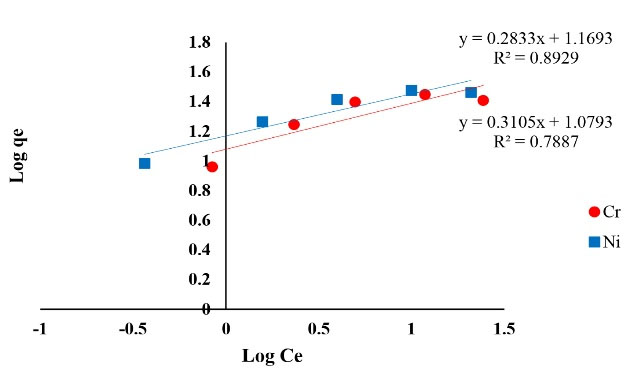
Figure 6.
The Plot of Freundlich Isotherm of Ni and Cr Adsorption.
.
The Plot of Freundlich Isotherm of Ni and Cr Adsorption.
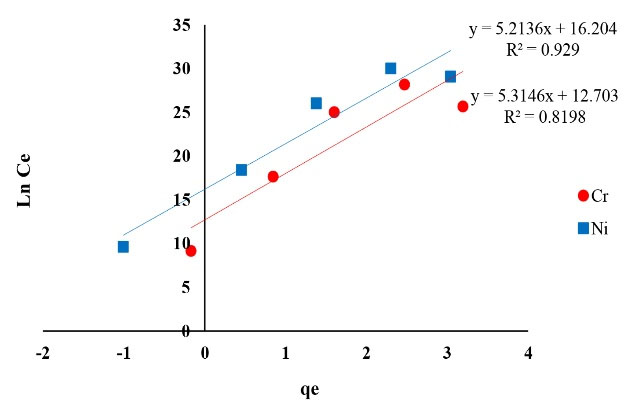
Figure 7.
The Plot of Temkin Isotherm of Ni and Cr Adsorption.
.
The Plot of Temkin Isotherm of Ni and Cr Adsorption.
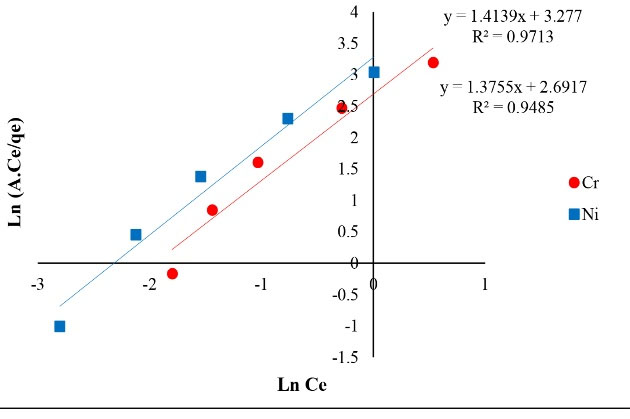
Figure 8.
The Plot of Redlich-Peterson Isotherm of Ni and Cr Adsorption.
.
The Plot of Redlich-Peterson Isotherm of Ni and Cr Adsorption.
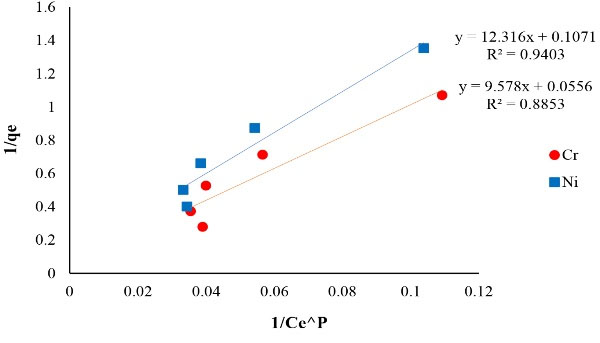
Figure 9.
The Plot of Koble-Corrigan Isotherm of Ni and Cr Adsorption.
.
The Plot of Koble-Corrigan Isotherm of Ni and Cr Adsorption.
Table 3.
Adsorption Modeling of Ni and Cr through Different Isotherms and Their Respective Parameters
|
Isotherms
|
Parameters
|
R
2
|
| Langmuir Ni |
qm= 30.48 (mg/g)
KL= 1.37 (L/mg) |
0.9982 |
| Langmuir Cr |
qm= 27.32 (mg/g)
KL= 1.07 (L/mg) |
0.992 |
| Freundlich Ni |
KF= 14.76 (mg/g (L/mg)1/n)
n= 3.52 |
0.8929 |
| Freundlich Cr |
KF= 12.003 (mg/g) (L mg-1)1/n)
n= 3.22 |
0.7887 |
| Temkin Ni |
BT= 5.213 (mg/g)
KT= 22.37 (L/mg) |
0.929 |
| Temkin Cr |
BT= 5.314 (mg/g)
KT= 10.91 (L/mg) |
0.8198 |
| Redlich-Peterson Ni |
g= 1.413
BRP= 26.49 (L/mg)
ARP= 1.6 (mg/g (L/mg)) |
0.9713 |
| Redlich-Peterson Cr |
g= 1.375
BRP= 14.75 (L/mg)
ARP= 1.8 (mg/g (L/mg)) |
0.9485 |
| Koble-Corrigan Ni |
AKC=0.081 (mg/g (L/mg))
BKC= 0.0086 (L/mg)P
P= 0.3 |
0.9403 |
| Koble-Corrigan Cr |
AKC=0.1044 (mg/g (L/mg))
BKC= 0.0058 (L/mg)P
P= 0.4 |
0.8853 |
The correlation coefficient achieved from isotherms assessment can be used as a suitable criterion. The results of isotherm models including Langmuir, Freundlich, Temkin, Redlich-Peterson and Koble-Corrigan showed a high correlation coefficient. The achieved correlation coefficient for Langmuir model for both of the metals (Ni and Cr) was over 0.99, therefore, Ni and Cr adsorption via copper oxide nanoparticles follows Langmuir model. The Langmuir isotherm is based on monolayer adsorption of uniformly adsorbed material on all surfaces of the adsorbent and assumes that the adsorbent surface has equal energy sites where each adsorbed molecule is assigned to a single site (12).
In the studies conducted by Onundi et al and Malarvizhi & Santhi on Ni removal, the adsorption data indicated a high conformity with the Langmuir isotherm that is in concurrence with the present study results (26,35). Likewise, the results of the present study at this stage are in line with the results of Gupta and Rastogi on the Cr removal that follows Langmuir model (36).
3.7. Evaluation of Adsorption Kinetics
Adsorption kinetics study of Ni and Cr was conducted using two kinetic models including pseudo first order and second order kinetic models. The kinetic adsorption curves have been shown in Figs. 10 and 11. The coefficients related to kinetic models are tabulated in Table 4.
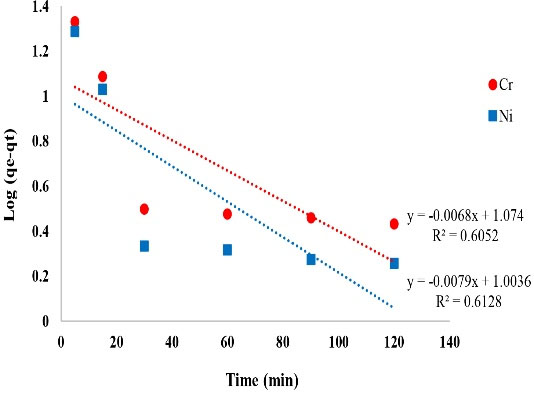
Figure 10.
The Plot of Pseudo First Order Adsorption Kinetics of Ni and Cr.
.
The Plot of Pseudo First Order Adsorption Kinetics of Ni and Cr.
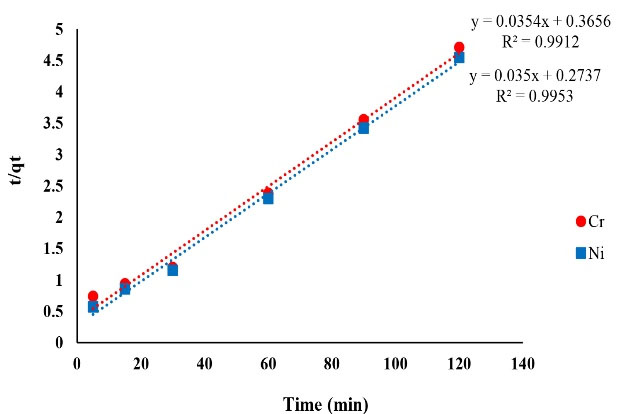
Figure 11.
The Plot of Pseudo Second Order Adsorption Kinetics of Ni and Cr.
.
The Plot of Pseudo Second Order Adsorption Kinetics of Ni and Cr.
Table 4.
Parameters of Adsorption Kinetic Models of Ni and Cr
|
Kinetic
|
Parameters
|
R
2
|
| Pseudo-first order Ni |
K1= 0.018 (1/h)
qe= 10.08 (mg/g) |
0.6128 |
| Pseudo-first order Cr |
K1= 0.015 (1/h)
qe= 11.85 (mg/g) |
0.6052 |
| Pseudo-second order Ni |
K2= 0.0044 (g/mg.h)
qe= 28.57 (mg/g) |
0.9953 |
| Pseudo-second order Cr |
K2= 0.0034 (g/mg.h)
qe= 28.24 (mg/g) |
0.9912 |
Based on the results of kinetic models and correlation coefficient of these models, it can be safely said that Ni and Cr adsorption on copper oxide nanoparticles was described better with pseudo second order kinetic model and their correlation coefficient values were over 0.99. In the pseudo-quadratic form, it is assumed that the chemical adsorption controls the adsorption phenomenon and the rate of occupation of the adsorption sites is proportional to the square of the number of sites not occupied. The results of a study entitled “removal of toxic hexavalent chromium from aqueous solutions by nickel ferrite-polyethylene nanocomposite” by Agrawal and Singh showed that pseudo second order kinetic model was suitable for Ni removal and the correlation coefficient value of this kinetic model was 0.998 which is in concurrence with the results of the present study (37). A study by Malkoc et al on the removal of Ni ions from aqueous solutions reported that the experimental data were fitted by the pseudo second order kinetic model (38).
3.8. Determination of Thermodynamic Parameters of Adsorption
The results of the effect of solution temperature on Ni and Cr removal efficiency are demonstrated in Fig. 12. In addition, the values of thermodynamic parameters of Ni and Cr removal have been tabulated in Table 5. Regarding the effect of solution temperature variations on Ni and Cr adsorption process, the results signified that the adsorption process was more favorable at higher temperatures, and this finding confirms that Ni and Cr adsorption process was endothermic. The positivity of ∆H values validates this fact and based on it, the increase in temperature causes an increase in maximum adsorption capacity. The positivity of ∆S values signifies an increase in the disorder on the solid-liquid surface during the adsorption process that causes a little change in the adsorbent structure and adsorbate, and as a result, the adsorption becomes irreversible (39). The negativity of ∆G values signifies that Ni and Cr ion adsorption is possible for the adsorbent such as copper oxide nanoparticles. The results of a study by Hubicki et a. are in concurrence with the result of the present study at this stage (40).
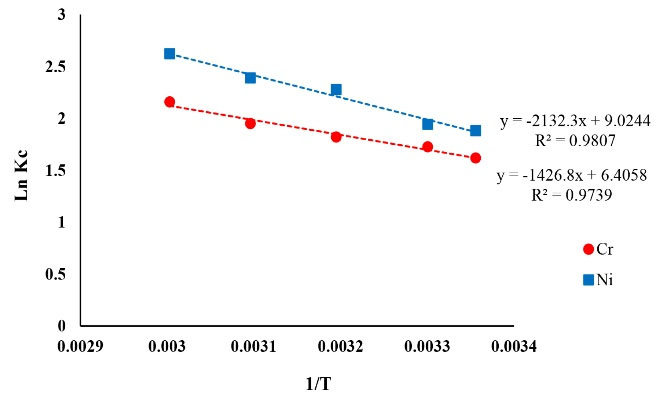
Figure 12.
The Plot of thermodynamic Parameters of Ni and Cr Adsorption.
.
The Plot of thermodynamic Parameters of Ni and Cr Adsorption.
Table 5.
The Calculated Thermodynamic Parameters for Ni and Cr ions Adsorption
|
Metal Ions
|
Thermodynamic Parameters
|
Temperature (°K)
|
R
2
|
| 298 |
303 |
313 |
323 |
333 |
| Ni |
ΔH (kJ/mol) |
|
|
17.727 |
|
|
0.9807 |
| ΔS (J/mol.k) |
|
|
0.075 |
|
|
| ΔG (kJ/mol) |
-4.65673 |
-4.8935 |
-5.92578 |
-6.41717 |
-7.26074 |
| Cr |
ΔH (kJ/mol) |
|
|
11.862 |
|
|
0.9739 |
| ΔS (J/mol.k) |
|
|
0.053 |
|
|
| ΔG (kJ/mol) |
-4.00998 |
-4.34935 |
-4.73544 |
-5.2346 |
-5.97493 |
4. Conclusion
In this research, the effect of various parameters on Ni and Cr adsorption from aqueous solutions via copper oxide nanoparticles was assessed. Based on the results of this study, it can be safely stated that copper oxide nanoparticles under optimal conditions could highly remove Ni and Cr with an initial concentration of 30 mg/L from aqueous solutions. The assessment of isotherm models revealed that the Langmuir model in comparison with the other adsorption models had a better conformity with the data. The evaluation of kinetic adsorption signified that the kinetic adsorption of Ni and Cr via iron oxide nanoparticles followed the pseudo second order model. Meanwhile, considering the achieved thermodynamic constants, it was clear that the adsorption process of metals viz. Ni and Cr via iron oxide nanoparticles was endothermic and spontaneous. In conclusion, the results of this study signified that copper oxide nanoparticles have a high ability in removing heavy ions viz Ni and Cr from the aqueous solutions. The research was conducted with an aim to assess the efficiency of copper oxide nanoparticles as an adsorbent to remove Ni and Cr. The effect of pH, adsorbent dosage, contact time, initial concentration of metals (Ni and Cr) on the adsorption rate was evaluated and removal of these elements from aqueous solutions was measured using Atomic Absorption Spectrum System (Conter AA700). Moreover, the kinetic and isotherm besides thermodynamic adsorption models were assessed. The highest Ni and Cr removal rate occurred at an optimal pH of 7, and an initial concentration of 30 mg/L, a time period of 30 minutes, and 1 g/L of copper oxide nanoparticles. In fact, with the increase of adsorbent dosage and contact time, the removal efficiency increased and with initial concentration increase of Ni and Cr ions, the removal efficiency reduced. The correlation coefficient of isotherm models viz. Langmuir, Freundlich, Temkin, Redlich-Peterson, and Koble-Corrigan showed that Ni and Cr adsorption via copper oxide nanoparticles better follows the Langmuir model in relation to other models. The results showed that kinetic adsorption of Ni and Cr via copper oxide nanoparticles follows the second order pseudo model with correlation coefficients above 0.99. In addition, the achieved thermodynamic constants revealed that the adsorption process of metals (i.e., Ni and Cr) via copper oxide nanoparticles was endothermic and spontaneous and the reaction enthalpy values for these metals were 17.727 and 11.862 kJ/mol, respectively. In conclusion, copper oxide nanoparticles can be used as effective and environmentally compatible adsorbents to remove Ni and Cr ions from the aqueous solutions.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
References
- Karimi P, Javanshir S, Sayadi MH, Arabyarmohammadi H. Arsenic Removal from Mining Effluents Using Plant-Mediated, Green-Synthesized Iron Nanoparticles. Processes 2019; 7(10):759. doi: 10.3390/pr7100759 [Crossref] [ Google Scholar]
- Saçmacı Ş, Kartal Ş, Saçmacı M. Determination of Cr (III), Fe (III), Ni (II), Pb (II) and Zn (II) ions by FAAS in environmental samples after separation and preconcentration by solvent extraction using a triketone reagent. Fresenius Environ Bull 2012; 21(6a):1563-70. [ Google Scholar]
- Sayadi MH, Sobhani S, Shekari H. Photocatalytic degradation of azithromycin using GO@ Fe3O4/ZnO/SnO2 nanocomposites. J Clean Prod 2019; 232:127-36. doi: 10.1016/j.jclepro.2019.05.338 [Crossref] [ Google Scholar]
- Yang G, Tang L, Lei X, Zeng G, Cai Y, Wei X. Cd (II) removal from aqueous solution by adsorption on α-ketoglutaric acid-modified magnetic chitosan. Appl Surf Sci 2014; 292:710-6. doi: 10.1016/j.apsusc.2013.12.038 [Crossref] [ Google Scholar]
- Arsiya F, Sayadi MH, Sobhani S. Arsenic (III) adsorption using palladium nanoparticles from aqueous solution. J Water Environ Nanotechnol 2017; 2(3):166-73. doi: 10.22090/jwent.2017.03.004 [Crossref] [ Google Scholar]
- Li F, Chen Y, Huang H, Cao W, Li T. Removal of rhodamine B and Cr (VI) from aqueous solutions by a polyoxometalate adsorbent. Chem Eng Res Des 2015; 100:192-202. doi: 10.1016/j.cherd.2015.05.030 [Crossref] [ Google Scholar]
- Taghipour M, Jalali M. Influence of organic acids on kinetic release of chromium in soil contaminated with leather factory waste in the presence of some adsorbents. Chemosphere 2016; 155:395-404. doi: 10.1016/j.chemosphere.2016.04.063 [Crossref] [ Google Scholar]
- Yazdani A, Sayadi Sayadi, MH MH, Heidari A. Green biosynthesis of palladium oxide nanoparticles using dictyota indica seaweed and its application for adsorption. J Water Environ Nanotechnol 2018; 3(4):337-47. doi: 10.22090/jwent.2018.04.006 [Crossref] [ Google Scholar]
- Sayadi MH, Shekari H. Biosorption of cadmium and lead from aqueous solutions using Spirogyra. J Environ Stud 2017; 43(3):379-90. doi: 10.22059/jes.2018.225238.1007380.[Persian] [Crossref] [ Google Scholar]
- Shekari H, Sayadi MH, Rezaei MR, Allahresani A. Synthesis of nickel ferrite/titanium oxide magnetic nanocomposite and its use to remove hexavalent chromium from aqueous solutions. Surfaces and Interfaces 2017; 8:199-205. doi: 10.1016/j.surfin.2017.06.006 [Crossref] [ Google Scholar]
- Zhou X, Jing G, Lv B, Zhou Z, Zhu R. Highly efficient removal of chromium (VI) by Fe/Ni bimetallic nanoparticles in an ultrasound-assisted system. Chemosphere 2016; 160:332-41. doi: 10.1016/j.chemosphere.2016.06.103 [Crossref] [ Google Scholar]
- Langmuir I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 1916; 38(11):2221-95. doi: 10.1021/ja02268a002 [Crossref] [ Google Scholar]
- Freundlich H. Über die adsorption in lösungen. Z Phys Chem 1907; 57(1):385-470. doi: 10.1515/zpch-1907-5723 [Crossref] [ Google Scholar]
- Temkin MI, Pyzhev V. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physicochimica URSS 1940; 12:327-56. [ Google Scholar]
- Nitzsche R, Gröngröft A, Kraume M. Separation of lignin from beech wood hydrolysate using polymeric resins and zeolites–Determination and application of adsorption isotherms. Sep Purif Technol 2019; 209:491-502. doi: 10.1016/j.seppur.2018.07.077 [Crossref] [ Google Scholar]
- Syafiuddin A, Salmiati S, Jonbi J, Fulazzaky MA. Application of the kinetic and isotherm models for better understanding of the behaviors of silver nanoparticles adsorption onto different adsorbents. J Environ Manage 2018; 218:59-70. doi: 10.1016/j.jenvman.2018.03.066 [Crossref] [ Google Scholar]
- Lagergren S. About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar 1898; 24(4):1-39. [ Google Scholar]
- Ho YS. Review of second-order models for adsorption systems. J Hazard Mater 2006; 136(3):681-9. doi: 10.1016/j.jhazmat.2005.12.043 [Crossref] [ Google Scholar]
- Harrache Z, Abbas M, Aksil T, Trari M. Thermodynamic and kinetics studies on adsorption of Indigo Carmine from aqueous solution by activated carbon. Microchem J 2019; 144:180-9. doi: 10.1016/j.microc.2018.09.004 [Crossref] [ Google Scholar]
- Cheng Z, Tan ALK, Tao Y, Shan D, Ting KE, Yin XJ. Synthesis and characterization of iron oxide nanoparticles and applications in the removal of heavy metals from industrial wastewater. Int J Photoenergy 2012; 2012:608298. doi: 10.1155/2012/608298 [Crossref] [ Google Scholar]
- Farooghi A, Sayadi MH, Rezaei MR, Allahresani A. An efficient removal of lead from aqueous solutions using FeNi3@ SiO2 magnetic nanocomposite. Surfaces and Interfaces 2018; 10:58-64. doi: 10.1016/j.surfin.2017.11.005 [Crossref] [ Google Scholar]
- Mobasherpour I, Salahi E, Pazouki M. Removal of divalent cadmium cations by means of synthetic nano crystallite hydroxyapatite. Desalination 2011; 266(1-3):142-8. doi: 10.1016/j.desal.2010.08.016 [Crossref] [ Google Scholar]
- Wang P, Lo IM. Synthesis of mesoporous magnetic gamma-Fe2O3 and its application to Cr (VI) removal from contaminated water. Water Res 2009; 43(15):3727-34. doi: 10.1016/j.watres.2009.05.041 [Crossref] [ Google Scholar]
- Xin X, Wei Q, Yang J, Yan L, Feng R, Chen G. Highly efficient removal of heavy metal ions by amine-functionalized mesoporous Fe3O4 nanoparticles. Chem Eng J 2012; 184:132-40. doi: 10.1016/j.cej.2012.01.016 [Crossref] [ Google Scholar]
- Yazdanbakhsh M, Tavakkoli H, Hosseini SM. Characterization and evaluation catalytic efficiency of La0 5Ca0 5NiO3 nanopowders in removal of reactive blue 5 from aqueous solution. Desalination 2011; 281:388-95. doi: 10.1016/j.desal.2011.08.020 [Crossref] [ Google Scholar]
- Onundi YB, Mamun AA, Khatib MFA, Ahmed YM. Adsorption of copper, nickel and lead ions from synthetic semiconductor industrial wastewater by palm shell activated carbon. Int J Environ Sci Technol 2010; 7(4):751-8. doi: 10.1007/BF03326184 [Crossref] [ Google Scholar]
- Altun T, Pehlivan E. Removal of Cr (VI) from aqueous solutions by modified walnut shells. Food Chem 2012; 132(2):693-700. doi: 10.1016/j.foodchem.2011.10.099 [Crossref] [ Google Scholar]
- Pashai Gatabi M, Milani Moghaddam H, Ghorbani M. Efficient removal of cadmium using magnetic multiwalled carbon nanotube nanoadsorbents: equilibrium, kinetic, and thermodynamic study. J Nanopart Res 2016; 18(7):189. doi: 10.1007/s11051-016-3487-x [Crossref] [ Google Scholar]
- Chen S, Yue Q, Gao B, Xu X. Equilibrium and kinetic adsorption study of the adsorptive removal of Cr (VI) using modified wheat residue. J Colloid Interface Sci 2010; 349(1):256-64. doi: 10.1016/j.jcis.2010.05.057 [Crossref] [ Google Scholar]
- Hong H, Jiang WT, Zhang X, Tie L, Li Z. Adsorption of Cr (VI) on STAC-modified rectorite. Appl Clay Sci 2008; 42(1-2):292-9. doi: 10.1016/j.clay.2008.01.015 [Crossref] [ Google Scholar]
- Feng Y, Gong JL, Zeng GM, Niu QY, Zhang HY, Niu CG. Adsorption of Cd (II) and Zn (II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chem Eng J 2010; 162(2):487-94. doi: 10.1016/j.cej.2010.05.049 [Crossref] [ Google Scholar]
- Kumar R, Bishnoi NR, Garima Garima, Bishnoi K. Biosorption of chromium (VI) from aqueous solution and electroplating wastewater using fungal biomass. Chem Eng J 2008; 135(3):202-8. doi: 10.1016/j.cej.2007.03.004 [Crossref] [ Google Scholar]
- Gupta S, Babu BV. Removal of toxic metal Cr (VI) from aqueous solutions using sawdust as adsorbent: Equilibrium, kinetics and regeneration studies. Chem Eng J 2009; 150(2-3):352-65. doi: 10.1016/j.cej.2009.01.013 [Crossref] [ Google Scholar]
- Mahdavi S, Jalali M, Afkhami A. Removal of heavy metals from aqueous solutions using Fe3O4, ZnO, and CuO nanoparticles. J Nanopart Res 2012; 14(8):846. doi: 10.1007/s11051-012-0846-0 [Crossref] [ Google Scholar]
- Malarvizhi TS, Santhi T. Removal of Ni (II) ions from aqueous solution onto lignite fired flyash. Res J Chem Environ 2013; 17(3):10-8. [ Google Scholar]
- Gupta VK, Rastogi A. Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 2009; 163(1):396-402. doi: 10.1016/j.jhazmat.2008.06.104 [Crossref] [ Google Scholar]
- Agrawal S, Singh NB. Removal of toxic hexavalent chromium from aqueous solution by nickel ferrite-polyaniline nanocomposite. Desalin Water Treat 2016; 57(38):17757-66. doi: 10.1080/19443994.2015.1086700 [Crossref] [ Google Scholar]
- Malkoc E, Nuhoglu Y. Nickel (II) adsorption mechanism from aqueous solution by a new adsorbent--Waste acorn of Quercus ithaburensis. Environ Prog Sustain Energy 2010; 29(3):297-306. doi: 10.1002/ep.10412 [Crossref] [ Google Scholar]
- Pimentel PM, Melo MAF, Melo DMA, Assunção ALC, Henrique DM, Silva CN Jr. Kinetics and thermodynamics of Cu (II) adsorption on oil shale wastes. Fuel Process Technol 2008; 89(1):62-7. doi: 10.1016/j.fuproc.2007.07.003 [Crossref] [ Google Scholar]
- Hubicki Z, Wolowicz A, Leszczynska M. Studies of removal of palladium (II) ions from chloride solutions on weakly and strongly basic anion exchangers. J Hazard Mater 2008; 159(2-3):280-6. doi: 10.1016/j.jhazmat.2008.02.017 [Crossref] [ Google Scholar]