Avicenna J Environ Health Eng. 6(1):41-48.
doi: 10.34172/ajehe.2019.06
Original Article
Optimization of Coagulation-Flocculation Process of Landfill Leachate by Tin (IV) Chloride Using Response Surface Methodology
Abdul Aziz Hamidi 1, 2, *  , Syed Zainal Sharifah Farah Fariza 1, Alazaiza Motasem Y.D 3
, Syed Zainal Sharifah Farah Fariza 1, Alazaiza Motasem Y.D 3
Author information:
1School of Civil Engineering, Engineering Campus, Universiti Sains Malaysia, 14300 Nibong Tebal, Penang, Malaysia
2Solid Waste Management Cluster, Science and Technology Research Centre, Engineering Campus, Universiti Sains Malaysia, 14300 Nibong Tebal, Penang, Malaysia
3Department of Civil Engineering, College of Engineering (COE), ASharqiyah University (ASU), 400 Ibra, Oman
*Correspondence to Hamidi Abdul Aziz, Tel: + 60-45996215, Fax: +60-45996906, E-mail:
cehamidi@usm.my
Abstract
Landfill leachate is highly polluted and generated as a result of water infiltration through solid waste produced domestically and industrially. This study investigated the applicability of the response surface methodology (RSM) to optimize the removal performances of chemical oxygen demand (COD), color, and suspended solids (SS) from landfill leachate by coagulation process using Tin tetrachloride pentahydrate. The leachate samples were collected from Alor Pongsu Landfill (APLS) in Perak, Malaysia. Before starting the experiments, general characterization was carried out for raw leachate samples to investigate their physical and chemical properties. The effects of the dosage and pH of SnCl4 on the removal performances were evaluated as well. An ideal experimental design was performed based on the central composite design (CCD) by RSM. In addition, this RSM was used to evaluate the effects of process variables and their interaction toward the attainment of their optimum conditions. The statistical design of the experiments and data analysis was resolved using the Design-Expert software. Further, the range of coagulant dosage and pH was selected based on a batch study which was conducted at 13000 mg/L to 17000 mg/L of SnCl4 and pH ranged from 6 to 10. The results showed that the optimum pH and dosage of SnCl4 were 7.17 and 15 g/L, respectively, where the maximum removal efficiency was 67.7% for COD and 100% for color and SS. The results were in agreement with the experimental data with a maximum removal efficiency of 67.84 %, 98.6 %, and 99.3%, for COD, color, and SS, respectively. Overall, this study verified that the RSM method was viable for optimizing the operational condition of the coagulation-flocculation process.
Keywords: Landfill, Leachate, Tin tetrachloride, Coagulation, Flocculation, RSM
Copyright and License Information
© 2019 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
Sanitary landfill is the common method for solid waste disposal in most developing countries due to its simple operation and cheap coast compared to the other methods (1). There are more than 230 landfills in Malaysia and quite a number are just dumpsites with minimum environmental protection (2). The old dumpsites still emit landfill leachate, which is stable and difficult to be further degraded biologically. Leachate is defined as the liquid generated when water percolates through solid waste and includes dissolved or suspended materials from different disposed materials. It often contains high-strength wastewater with high pH, chemical oxygen demand (COD), biochemical oxygen demand (BOD), inorganic salts, and toxicity. Leachate characteristics play significant roles in determining suitable leachate treatment methods due to its constituents that may change with time. Biological processes are appropriate to young leachate compared to older or stabilized leachate due to its high molecular weight organics and consist of low COD (3). Hence, the coagulation-flocculation process is one of the frequently applied techniques for stabilized or old leachate.
In addition, the purification of aqueous colloidal pollutants is achievable by coagulation-flocculation techniques. In the coagulation process, the destabilization of colloidal suspensions occurs by neutralizing electric forces amidst suspended particles while flocculation gains the particles bridging each other and encourages forming flocs or larger aggregates (4). The efficiency of the coagulation and flocculation process consists of the type and dosage of coagulant and flocculants, pH, mixing speed and time, as well as temperature and retention time (5). The proper combination of these factors is advantageous for obtaining high efficiency of treatment. The coagulation-flocculation process is widely applied in water and wastewater treatments. However, it involves a time-consuming process with a try and error approach where optimum conditions are determined at different experimental conditions such as the effect on pH, dosages, mixing speed, and the like (6,7). As an alternative to this conventional method, the response surface methodology (RSM) technique was applied in the design of an experiment which allows a restricted number of experiments, hence reducing the time for conducting the experiments. RSM is a tool for identifying the influences of factors and their responsive operation (8). This tool involves a statistical, mathematical technique which indicates the empirical design (9-12).Therefore, it is important to choose an experimental design that suits an appropriate mathematical function for evaluating the quality of the model and predicting its accuracy in relation to the obtained experimental data.
Inorganic coagulants such as aluminum (alum) sulfate, ferrous sulfate, ferric chloride, polyaluminium chloride, and ferric chloro-sulfate are usually used in the coagulation-flocculation process (13-17). Most of these coagulants consist of the trivalent state of coagulant. Generally, the effectiveness of charge neutralization is greater when the cation charge is greater. To improve the treatment of water and wastewater, several studies were conducted to find the potential of four valence state chemicals. For instance, Aziz et al (18) applied titanium tetrachloride (TiCl4) as a coagulant to treat semi-aerobic leachate and Hussain et al (19) used Zirconium (IV) chloride as a coagulant to treat the surface water.
Tin tetrachloride (SnCl4) is one of the inorganic tin compounds, which is also a part of Lewis acid. This acid is a compound or ionic species such as H+ that can accept an electron pair from a donor compound. Since Lewis acid is able to accept an electron, it was studied and used as a coagulant in the treatment. Some Lewis acids are TiCl4, ferric chloride, aluminum chloride, zinc chloride, and SnCl4.
Recently, many of these compounds have been used as a coagulant for the coagulation-flocculation process. Pushpalatha and Lokeshappa (20) utilized TiCl4 as a coagulant to treat semi-aerobic leachate. In addition, Galloux et al (21) used polytitanium tetrachloride for coal-mining wastewater treatment and concluded that the effectiveness of charge neutralization is parallel to the charge of the cation. In other words, the greater the cation charge, the greater is the effectiveness of charge neutralization. For example, the relative effectiveness of monovalent (Na+), divalent (Ca2+), and trivalent (Al3+) ions is 1:60:700, respectively. It shows that the trivalent of aluminum ions is 700 times more effective regarding charge neutralization compared to monovalent ion (22).
Considering the above-mentioned discussions, this paper aimed to study the applicability of RSM to investigate the potential use of SnCl4 as a coagulant for treating landfill leachate. Further, the RSM was applied to determine the optimum operational condition in terms of dosage and pH on the removal of COD, color, and suspended solids (SS).
2. Materials and Methods
2.1. Leachate Sampling
Alor Pongsu Landfill (APLS) or Beriah Landfill in Perak was constructed as open dumping, which started its operation since 2000.
As shown in Fig. 1, it is located at 5°05’14.20” N and 100°36’10.53” E and approximately covers an area of 8 acres within palm oil plantation. Furthermore, the site is positioned on an area of alluvium deposits which consist of silt, clay, sand, and gravel (23,24). Moreover, it receives an average of 660 000 metric tons of domestic solid waste per year which represents the daily average of 200 metric tons. The climate of this region is classified as a typical Malaysian Peninsula (equatorial) with a uniform daily temperature of a minimum and maximum of 30°C-40°C and high humidity of 80%-90%. This area also receives a high average rainfall of about 1800 mm (1).
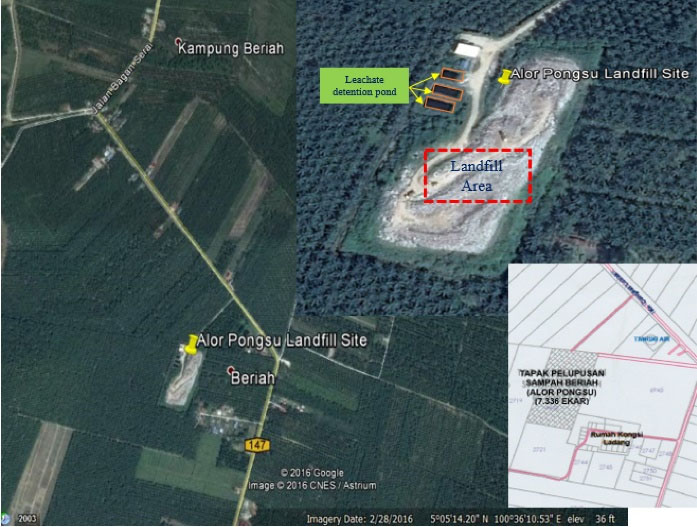
Figure 1.
Alor Pongsu Landfill Site.
Source. Google Map (2016) and Majlis Daerah Kerian, Perak, Malaysia.
.
Alor Pongsu Landfill Site.
Source. Google Map (2016) and Majlis Daerah Kerian, Perak, Malaysia.
Raw landfill leachate samples from APLS were collected at leachate detention pond by using 10 L and 20 L HDPE bottles. Monthly sampling was conducted between April 2016 and October 2018. The samples were then kept in cold storage at 4°C in accordance with the Standard Methods for the Examination of Water and Wastewater (25). Before each experiment, the samples were conditioned at ambient temperature for about two hours before the jar test. The in-situ parameters were also taken using a YSI 556 multi-probe by dipping the instrument into the leachate pond. The characteristics of raw leachate are provided in Table 1.
Table 1.
The Raw Leachate Characteristics
|
Parameter
|
Unit
|
Discharge Limit for Leachate
a
|
Minimum
|
Maximum
|
Average
b
|
| pH |
- |
6.0-9.0 |
7.52 |
8.15 |
7.84 |
| Temperature |
°C |
40.00 |
26.67 |
31.72 |
29.20 |
| Turbidity |
NTU |
- |
228.75 |
337.50 |
306.25 |
| SS |
mg/L |
50 |
591.67 |
866.67 |
745.00 |
| Color |
Pt Co. |
100 |
16,389 |
23,020 |
19,705 |
| COD |
mg/L |
400 |
3,080 |
5,323 |
4,202 |
| BOD5 |
mg/L |
20 |
28 |
236 |
132 |
| BOD5/COD |
- |
- |
0.01 |
0.07 |
0.04 |
| NH3-N |
mg/L |
5 |
870 |
1,850 |
1,360 |
| Conductivity |
S/cm |
- |
13,720 |
17,717 |
15,719 |
| DO |
mg/L |
- |
0.04 |
1.10 |
0.57 |
| TDS |
mg/L |
- |
4,855 |
8,110 |
6,483 |
Abbreviations: SS, suspended solids; COD, chemical oxygen demand; BOD, biochemical oxygen demand; DO, dissolved oxygen; TDS, total dissolved solids.
aPermissible condition for the discharge of leachate by Environmental Quality (Control of Pollution from Solid Waste Transfer Station and Landfill) Regulations, 2009 (PU(A) 433) Regulation 13; bAverage value for nine samples taken from April 2016 to October 2018.
The landfill can be considered as anaerobic landfill leachate with COD, BOD5, color, SS, and NH3-N exceeded the permissible discharge limits of leachate effluent in accordance with the Environmental Quality Regulation (2009). The BOD5/COD of 0.04 (<0.1) ratio indicates that the leachate is old and stabilized since the landfill has been in operation for more than 10 years (26). The leachate is dark in the color intensity of 19,705 Pt-Co and is slightly alkaline (pH =8). The presence of humic substances mainly contributes to the dark color. As the leachate is stable, a lower concentration of volatile fatty acids is expected which increases the pH (1,26).
The coagulation-flocculation process was performed using standard jar test equipment (VELP– Scientific, Model JLT6, Italy). The applied coagulant was tin tetrachloride pentahydrate (SnCl4.5H2O) in granular form and was prepared as a stock solution before the experiment. The pH of the leachate samples was adjusted by using 3M HCL and 3M NaOH. Six beakers with 1L volume were filled with 500 mL of leachate and later, a pre-determined concentration of coagulant was added, and finally, examined at a pH of 8. Rapid mixing for all experiments was set at the speed of 250 rpm for 4 minutes (3), followed by a slow mixing speed at 40 rpm for 30 minutes. Then, it was allowed to settle for 30 minutes and the retention time was set at 4 hours.
2.2. Design of Experiment and Analysis
The statistical design of the experiments and data analysis was resolved using the Design-Expert software (version 6.0.6). This software was also used to display the 3D surface and 2D contour of the response surface. In this study, the central composite design (CCD) and RSM were used to optimize and evaluate the correlation between two independent variables including pH and dosage (Table 2). CCD is an experimental design, which is useful in RSM, for building a second-order (quadratic) model for the response variable without needing to use a complete three-level factorial experiment. The range of coagulant dosage and pH was selected based on a batch study which was conducted at 13 000 mg/L to 17 000 mg/L of SnCl4 and pH ranged from 6 to 10. Moreover, dependent factors included COD, SS, and color removals. The performance of the process was evaluated by using a quadratic model of (equation 1). Eventually, the total number of the achieved experiments from these factors was 13 CCD experimental design with 5 replicates at a central point.
Table 2.
Design Summary for Response and Factor
|
Response
|
Minimum
|
Maximum
|
| COD removal |
19.86 |
67.54 |
| Color removal |
46.69 |
99.60 |
| SS removal |
49.32 |
99.40 |
| Factor |
Low Actual |
High Actual |
| pH |
6 |
10 |
| Dosage (mg/L) |
13000 |
17000 |
Abbreviations: COD, chemical oxygen demand; SS, suspended solids.
where Y is the response and Xi and Xj are the variables. Similarly, βdenotes the regression coefficient and k represents the number of factors that were studied and optimized in the experiment. Finally, e indicates the random error.
As shown in Table 3, the perceived percent of removal efficiencies differed between 19.86% and 67.54%, 46.69% and 99.60%, as well as 49.32% and 99.40% for COD, color, and SS, respectively.
Table 3.
Factor and Response to the Experimental Condition
|
Run No.
|
Factor A pH
|
Factor B Dosage (mg/L)
|
Response 1 COD Removal (%)
|
Response 2 Color Removal (%)
|
Response 3 SS Removal (%)
|
| 1 |
10.00 |
13,000 |
19.86 |
46.69 |
49.32 |
| 2 |
8.00 |
15,000 |
62.05 |
97.98 |
99.15 |
| 3 |
8.00 |
17,000 |
60.73 |
99.35 |
99.40 |
| 4 |
6.00 |
15,000 |
61.13 |
99.60 |
97.15 |
| 5 |
6.00 |
13,000 |
54.18 |
96.60 |
96.44 |
| 6 |
8.00 |
15,000 |
67.54 |
98.00 |
98.66 |
| 7 |
8.00 |
15,000 |
62.05 |
98.25 |
99.15 |
| 8 |
8.00 |
15,000 |
67.54 |
98.05 |
99.01 |
| 9 |
8.00 |
13,000 |
59.95 |
90.98 |
94.35 |
| 10 |
10.00 |
15,000 |
30.06 |
57.45 |
55.82 |
| 11 |
6.00 |
17,000 |
61.77 |
97.59 |
94.65 |
| 12 |
10.00 |
17,000 |
32.65 |
61.98 |
60.23 |
| 13 |
8.00 |
15,000 |
62.05 |
98.21 |
99.01 |
Abbreviations: COD, chemical oxygen demand; SS, suspended solids.
3. Results and Discussion
Analysis of variance (ANOVA) was applied to achieve the correlation between the process variables and the responses whereas experimental data were used to verify the adequacy of the used model (12,27). The quality of the fit polynomial model was expressed by the value of the correlation coefficient (R2) and its statistical significance was checked by the F test in the same program (6). The R-squared value indicated the correlation coefficient for the model while the predicted R-squared was used to determine the accuracy of the model to predict the response values.
The ANOVA results are presented in Table 4. The quadratic models were significant at P<0.05. The obtained value for R2 is 0.9776, 0.9999, and 0.9997 for COD, color, and SS, respectively. An adequate precision (AP) differentiates the range of the predicted values at the design points to the average prediction errors. The AP value for COD is 20.488, followed by 477.580 and 222.377 for color and SS. According to Saha et al (28), AP value greater than 4 is adequate and can be used to operate the design space interpreted by the CCD. The corresponding model and the coefficient are considered more significant when P is more than F-value (29).
Table 4.
ANOVA Results and Quadratic Model for COD, Color, and SS Removals
|
Response
|
Source
|
Sum of Squares
|
Degree of Freedom
|
Mean Square
|
F-value
|
Prob >F
|
Remarks
|
| COD removal |
Model |
2881.53 |
5 |
576.31 |
61.17 |
<0.0001 |
SD = 3.07,
CV = 5.69,
R2 = 0.9776,
Adj. R2 = 0.9616,
Adeq precision = 20.488 |
| A |
1488.69 |
1 |
1488.69 |
158.01 |
<0.0001 |
| B |
74.62 |
1 |
74.62 |
7.92 |
0.0260 |
| A2 |
939.68 |
1 |
939.68 |
99.74 |
<0.0001 |
| B2 |
37.82 |
1 |
37.82 |
4.01 |
0.8520 |
| AB |
6.76 |
1 |
6.76 |
0.72 |
0.4250 |
| Residual |
65.95 |
7 |
9.42 |
|
|
| Lack of fit |
29.78 |
3 |
9.93 |
1.10 |
0.4469 |
| Pure error |
36.17 |
4 |
9.04 |
|
|
| Color removal |
Model |
4263.92 |
5 |
852.78 |
27949.62 |
<0.0001 |
SD = 0.17,
CV = 0.20,
R2 = 0.9999,
Adj. R2 = 0.9999,
Adeq precision = 447.580 |
| A |
2716.60 |
1 |
2716,60 |
89035.46 |
<0.0001 |
| B |
101.27 |
1 |
101.27 |
3319.09 |
<0.0001 |
| A2 |
1051.68 |
1 |
1051.68 |
34468.39 |
<0.0001 |
| B2 |
22.81 |
1 |
22.81 |
747.49 |
<0.0001 |
| AB |
51.12 |
1 |
51.12 |
1675.52 |
<0.0001 |
| Residual |
0.21 |
7 |
0.031 |
|
|
| Lack of fit |
0.15 |
3 |
0.051 |
3.30 |
0.1395 |
| Pure error |
0.061 |
4 |
0.015 |
|
|
| SS removal |
Model |
4276.06 |
5 |
855.21 |
7652.77 |
<0.0001 |
SD = 0.33,
CV= 0.38,
R2 = 0.9998,
Adj. R2 = 0.9997,
Adeq precision = 222.377 |
| A |
2516.17 |
1 |
2516.17 |
22515.71 |
<0.0001 |
| B |
33.46 |
1 |
33.46 |
299.46 |
<0.0001 |
| A2 |
1352.21 |
1 |
1352.21 |
12100.08 |
<0.0001 |
| B2 |
8.33 |
1 |
8.33 |
94.54 |
<0.0001 |
| AB |
40.32 |
1 |
40.32 |
360.82 |
<0.0001 |
| Residual |
0.70 |
7 |
0.11 |
|
|
| Lack of fit |
0.62 |
3 |
0.21 |
5.16 |
0.0735 |
| Pure error |
0.16 |
4 |
0.040 |
|
|
Abbreviations:ANOVA, analysis of variance; COD, chemical oxygen demand; SS: suspended solids; SD, standard deviation; CV, coefficient of variance; R2, the value of correlation coefficient.
According to Malakahmad and Chuan (8), the low standard deviation shows that the quadratic model is the best and the coefficient of variance (CV) of less than 10% signifies that the model is reproducible. In addition, CV is the ratio of the standard error of estimate to the mean value of the observed response which defines the reproducibility of the model. The values of CV are 5.69, 0.20, and 0.38 for COD, color, and SS, respectively, and they are considered reproducible. The plots of the standardized residual for COD, color, and SS are shown in Fig. 2. The normal probability plots help to evaluate the models. The point follows the straight line for each case when the residuals follow a normal distribution, which may describe a normal probability plot. Thus, these experimental data are acceptable. Table 5 presents the final regression model in terms of their coded and actual factors.
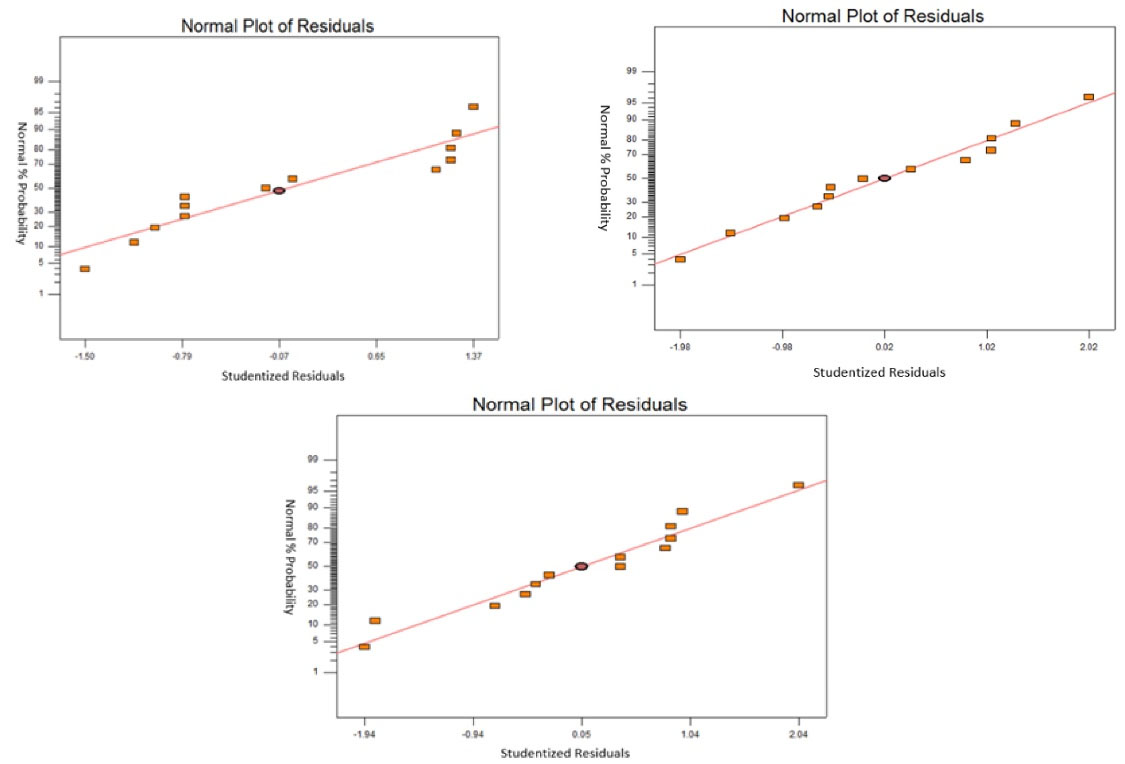
Figure 2.
Normal Probability Plot of Standardized Residual for Chemical Oxygen Demand (a), Color (b), and Suspended Solids (c)
.
Normal Probability Plot of Standardized Residual for Chemical Oxygen Demand (a), Color (b), and Suspended Solids (c)
Table 5.
Final Equations in Terms of Coded and Actual Factors
|
|
Final Equation in Terms of Coded Factors
|
Final Equation in Terms of Actual Factors
|
| COD removal |
+ 64.19-15.75A + 3.53B-18.45A2- 3.70B2+1.30AB |
-363.52601 + 61.03055A + 0.026916B-4.61134A2- 9.2508E-007B2 + 3.25000E-004AB |
| Color removal |
+ 98.08-21.28A + 4.11B-19.51A2- 2.87B2+3.58AB |
-214.22723 + 54.00907A + 0.016456B-4.87841A2-7.18405E-007B2 + 8.9375E-004AB |
| SS removal |
+ 98.89-20.48A + 2.36B-22.13A2- 1.74B2+3.18AB |
-193.38128 + 66.36148A + 7.85626E-003B-5.53168A2- 4.3418E-007B2 + 7.93750E-004AB |
Abbreviations: COD, chemical oxygen demand; SS, suspended solids.
Fig. 3 illustrates the 3D response surface plots and 2D contour plots of the quadratic model in terms of COD, color, and SS removals, respectively. The purpose was to estimate the correlation relationship between independent variables and the responses of the models. The maximum observed removal of COD was 68.1%, and a complete reduction (100%) was observed in color and SS.
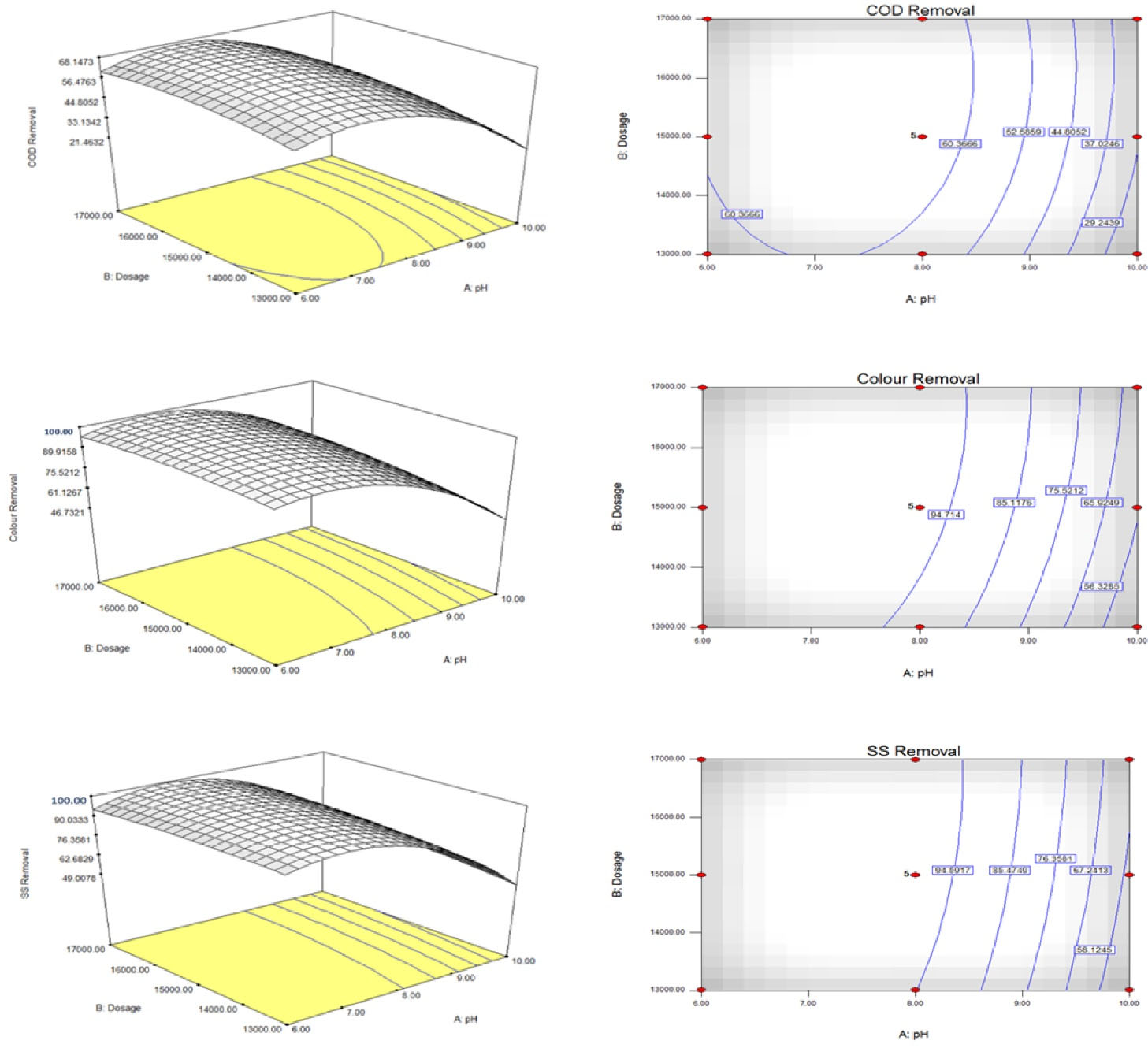
Figure 3.
Response Surface and Contour Plots for COD (a), color (b), and SS (c)
.
Response Surface and Contour Plots for COD (a), color (b), and SS (c)
There were 10 solutions given in the RSM optimization process. As shown in Table 6, the solution No. 6 was chosen due to its best performance. To confirm the validity of the statistical experimental strategies, a verification experiment was run at optimum conditions determined based on RSM solutions. The samples were stirred at a constant speed of 250 rpm for 4 minutes. This was followed by slow mixing at 40 rpm for 30 minutes to encourage collisions between destabilization colloidal particles (30) before settlement within 30 minutes. The removal efficiencies were observed as 67.84%, 98.6%, and 99.3% for COD, color, and SS, respectively. This indicates the experimental results were almost identical with the predicted RSM values, which confirmed that the RSM application was relevant for optimizing the operational condition of the coagulation-flocculation process. Therefore, the optimum value was at a pH of 7.17 and Tin tetrachloride (SnCl4) dosage of 15,012 mg/L. The results are in agreement with those of the study conducted by Ghafari et al (13), in which the RSM was applied to optimize the coagulation-flocculation treatment of leachate using poly-aluminum chloride and alum. They concluded that the data and model predictions agreed well where COD, turbidity, color, and TSS removal efficiencies of 43.1%, 94.0%, 90.7%, and 92.2% were demonstrated for polyaluminium chloride, as well as 62.8%, 88.4%, 86.4%, and 90.1% for alum. In another study, Bashir et al (31) used RSM to optimize the treatment of stabilized sanitary landfill leachate using the anionic resin. Based on their results, the color, COD, SS, and turbidity removal efficiencies of 91.5%, 70.3%, 93.1%, and 92.4% were experimentally attained and were found to fit well with the prediction model. Accordingly, the RSM is considered as a good tool for the optimization of landfill leachate using different treatment methods.
Table 6.
Optimization Results According to RSM and the Verified Result Based on Laboratory Experimental
|
|
Solution
|
Dosage (mg/L)
|
pH
|
COD Removal (%)
|
Color Removal (%)
|
SS Removal (%)
|
| RSM |
No. 6 |
15,011.60 |
7.17 |
67.56 |
100 |
100 |
| Lab. experiment |
- |
15,000 |
8 |
67.74 |
98.57 |
99.29 |
Abbreviations: RSM, response surface methodology; COD, chemical oxygen demand; SS, suspended solids.
4. Conclusion
The present study investigated the applicability of the RSM tool in order to observe the removal efficiency of COD, color, and SS using SnCl4 as a coagulant in the coagulant-flocculation process. The optimum pH was 7.17 for SnCl4 with an optimum dosage of 15 012 mg/L. The results revealed that the SnCl4 was capable of removing all color and SS from the landfill leachate while it only removed 67.6% of the COD. In addition, the RSM results were compared with the experimental data and both results were consistent where the obtained COD, color, and SS removals were 67.84%, 98.6%, and 99.3%, respectively. Overall, the results of this study demonstrated that the RSM application was appropriate for optimizing the operational condition of the coagulation- flocculation process.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
Acknowledgement
This work was funded by Universiti Sains Malaysia under RUI Grant Scheme (Grant No. 1001/PAWAM/8014081) and RU bridging grant (304/PAWAM/6316096) for research associated with the Solid Waste Management Cluster, Engineering Campus, Universiti Sains Malaysia.
References
- Adhikari B, Khanal SN. Qualitative study of landfill leachate from different ages of landfill sites of various countries including Nepal. IOSR J Environ Sci Toxicol Food Technol 2015; 9(1):23-36. doi: 10.9790/2402-09132336 [Crossref] [ Google Scholar]
- Aziz SQ, Aziz HA, Yusoff MS, Bashir MJ, Umar M. Leachate characterization in semi-aerobic and anaerobic sanitary landfills: a comparative study. J Environ Manage 2010; 91(12):2608-14. doi: 10.1016/j.jenvman.2010.07.042 [Crossref] [ Google Scholar]
- Zainol NA, Aziz HA, Yusoff MS, Umar M. The use of polyaluminum chloride for the treatment of landfill leachate via coagulation and flocculation processes. Res J Chem Sci 2011; 1(3):34-9. [ Google Scholar]
- Bratby J. Coagulation and flocculation in water and wastewater treatment. IWA Publishing; 2016.
- Altaher H, ElQada E, Omar W. Pretreatment of wastewater streams from petroleum/petrochemical industries using coagulation. Advances in Chemical Engineering and Science 2011; 1(4):245-51. doi: 10.4236/aces.2011.14035 [Crossref] [ Google Scholar]
- Dawood A, Li Y. Modeling and optimization of new flocculant dosage and pH for flocculation: removal of pollutants from wastewater. Water 2013; 5(2):342-55. doi: 10.3390/w5020342 [Crossref] [ Google Scholar]
- Usefi S, Asadi-Ghalhari M. Modeling and Optimization of the Coagulation–Flocculation Process in Turbidity Removal from Aqueous Solutions Using Rice Starch. Pollution 2019; 5(3):623-36. doi: 10.22059/poll.2019.271649.552 [Crossref] [ Google Scholar]
- Malakahmad A, Chuan SY. Application of response surface methodology to optimize coagulation–flocculation treatment of anaerobically digested palm oil mill effluent using alum. Desalin Water Treat 2013; 51(34-36):6729-35. doi: 10.1080/19443994.2013.791778 [Crossref] [ Google Scholar]
- Carley KM, Kamneva NY, Reminga J. Response surface methodology: CASOS technical report. Center for Computational Analysis of Social and Organizational Systems, Carnegie Mellon University; 2004.
- Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008; 76(5):965-77. doi: 10.1016/j.talanta.2008.05.019 [Crossref] [ Google Scholar]
- Mosaddeghi MR, Pajoum Shariati F, Vaziri Yazdi SA, Nabi Bidhendi G. Application of response surface methodology (RSM) for optimizing coagulation process of paper recycling wastewater using Ocimum basilicum. Environ Technol. 2018:1-9. 10.1080/09593330.2018.1491637 .
- Abu Amr SS, Aziz HA, Bashir MJK. Application of response surface methodology (RSM) for optimization of semi-aerobic landfill leachate treatment using ozone. Appl Water Sci 2014; 4(3):231-9. doi: 10.1007/s13201-014-0156-z [Crossref] [ Google Scholar]
- Ghafari S, Aziz HA, Isa MH, Zinatizadeh AA. Application of response surface methodology (RSM) to optimize coagulation-flocculation treatment of leachate using poly-aluminum chloride (PAC) and alum. J Hazard Mater 2009; 163(2-3):650-6. doi: 10.1016/j.jhazmat.2008.07.090 [Crossref] [ Google Scholar]
- Farajnezhad H, Gharbani P. Coagulation treatment of wastewater in petroleum industry using poly aluminum chloride and ferric chloride. International Journal of Research and Reviews in Applied Sciences 2012; 13(1):306-10. [ Google Scholar]
- Lee CS, Robinson J, Chong MF. A review on application of flocculants in wastewater treatment. Process Saf Environ Prot 2014; 92(6):489-508. doi: 10.1016/j.psep.2014.04.010 [Crossref] [ Google Scholar]
- Rusdizal N, Aziz HA, Fatehah MO. Potential use of polyaluminium chloride and tobacco leaf as coagulant and coagulant aid in post-treatment of landfill leachate. Avicenna J Environ Health Eng 2015; 2(2):e5836. doi: 10.17795/ajehe-5836 [Crossref] [ Google Scholar]
- Kamaruddin MA, Abdullah MMA, Yusoff MS, Alrozi R, Neculai O. Coagulation-flocculation process in landfill leachate treatment: focus on coagulants and coagulants aid. IOP Conf Ser Mater Sci Eng 2017; 209(1):012083. doi: 10.1088/1757-899x/209/1/012083 [Crossref] [ Google Scholar]
- Aziz HA, Rosli MY, Abu Amr SS, Hussain S. Potential use of Titanium Tetrachloride as Coagulant to Treat Semi Aerobic Leachate Treatment. Aust J Basic Appl Sci 2015; 9(94):37-44. [ Google Scholar]
- Hussain S, van Leeuwen J, Chow CWK, Aryal R, Beecham S, Duan J. Comparison of the coagulation performance of tetravalent titanium and zirconium salts with alum. Chem Eng J 2014; 254:635-46. doi: 10.1016/j.cej.2014.06.014 [Crossref] [ Google Scholar]
- Pushpalatha TN, Lokeshappa B. The Use of Alum, Ferric Chloride and Titanium tetrachloride as Coagulants in Treating Landfill Leachate. International Journal of Science, Engineering and Technology Research (IJSETR) 2015; 4(6):2093-2096. [ Google Scholar]
- Galloux J, Chekli L, Phuntsho S, Tijing LD, Jeong S, Zhao YX. Coagulation performance and floc characteristics of polytitanium tetrachloride and titanium tetrachloride compared with ferric chloride for coal mining wastewater treatment. Sep Purif Technol 2015; 152:94-100. doi: 10.1016/j.seppur.2015.08.009 [Crossref] [ Google Scholar]
- Engelhardt TL. Coagulation, Flocculation and Clarification of Drinking Water. Loveland, Colorado: Hach Company; 2014.
- Zawawi MH. Shallow groundwater contamination study at landfill sites using integrated geophysical hydrochemistry and isotope hydrology techniques [Thesis]. Malaysia: Universiti Sains Malaysia; 2014.
- Syafalni S, Zawawi MH, Abustan I. Isotopic and hydrochemistry fingerprinting of leachate migration in shallow groundwater at controlled and uncontrolled landfill sites. World Appl Sci J 2014; 31(6):1198-206. doi: 10.5829/idosi.wasj.2014.31.06.217 [Crossref] [ Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater. 21th ed. Washington, DC: APHA; 2005.
- Bhalla B, Saini MS, Jha MK. Effect of age and seasonal variations on leachate characteristics of municipal solid waste landfill. Int J Res Eng Technol 2013; 2(8):223-32. doi: 10.15623/ijret.2013.0208037 [Crossref] [ Google Scholar]
- Talebi A, Teng TT, Alkarkhi AFM, Norli I, Low LW. Optimization of nickel removal using liquid–liquid extraction and response surface methodology. Desalin Water Treat 2012; 47(1-3):334-40. doi: 10.1080/19443994.2012.696432 [Crossref] [ Google Scholar]
- Saha PD, Dey A, Marik P. Batch removal of chromium (VI) from aqueous solutions using wheat shell as adsorbent: process optimization using response surface methodology. Desalin Water Treat 2012; 39(1-3):95-102. doi: 10.1080/19443994.2012.669164 [Crossref] [ Google Scholar]
- Trinh TK, Kang LS. Response surface methodological approach to optimize the coagulation–flocculation process in drinking water treatment. Chem Eng Res Des 2011; 89(7):1126-35. doi: 10.1016/j.cherd.2010.12.004 [Crossref] [ Google Scholar]
- Taşdemir T, Taşdemir A. Effect of mixing conditions on flocculation. In Proceedings of XIIIth International Mineral Processing Symposium; 2012. p. 831-7.
- Bashir MJK, Aziz HA, Yusoff MS, Aziz SQ, Mohajeri S. Stabilized sanitary landfill leachate treatment using anionic resin: Treatment optimization by response surface methodology. J Hazard Mater 2010; 182(1):115-22. doi: 10.1016/j.jhazmat.2010.06.005 [Crossref] [ Google Scholar]