Avicenna J Environ Health Eng. 6(1):55-65.
doi: 10.34172/ajehe.2019.08
Review Article
The Relevance of Isotherm and Kinetic Models to Chlorophenols Adsorption: A Review
Zaharaddeen N. Garba 1, * 
Author information:
1Department of Chemistry, Ahmadu Bello University Zaria, Nigeria
Abstract
The derivatives of phenols are among the most widely used chemicals in day-to-day life, which lead to water contamination by chlorophenols (CPs). These compounds belong to a class of those widely used chemicals that increase global concern about environmental protection due to their recalcitrant nature. Adsorption process has been employed for the removal of CPs from contaminated water out of many methods of wastewater treatment. This is due to its insensitivity to toxic substances, effectiveness, universal nature, fast kinetics, as well as the ease of operation and its simplicity in the design and applicability. Thus, this study compared the adsorption isotherm models such as linear and nonlinear and well discussed the fundamental characteristics, modelling, and mathematical derivations. Finally, the study highlighted and addressed the role of different isotherm models that were used in describing the adsorptive removal of CPs using various adsorbents.
Keywords: Chlorophenols, Adsorption, Isotherms, Linear, Nonlinear, Adsorbents
Copyright and License Information
© 2019 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
The importance of water to life cannot be overemphasized since it serves many other purposes apart from drinking and agriculture (1). A larger fraction of the earth is covered with water. Despite its abundant on the surface of the earth, the World Health Organization (WHO) only recommended a small portion of the fraction to be appropriate for drinking purposes. Even by considering the WHO recommendation, the majority of the populace in developing countries are known for the inadequate supply of hygienic drinking water (2). Natural and artificial activities are among numerous factors that contribute to water pollution which is among the most troubling environmental challenges bedevilling several countries. Water pollution occurs when substances that negatively affect living species contaminate the oceans, rivers, lakes, bays, and streams (3).
Chlorophenols (CPs) that are covalently bonded to one, two, three, four and/or five chlorine atoms are named as monochlorophenols, dichlorophenols, trichlorophenols, tetrachlophenols, and/or pentachlorophenols, respectively (4). They are also known as important pollutants, which are widely found in industrial wastewaters and characterized by low biodegradability, strong odour, persistency in the environment, along with carcinogenic and toxic features affecting human and its environment. Exposure to CPs affects human nervous and respiratory systems and makes them hazardous to health (5-8). Table 1 lists some properties of selected CPs.
Table 1.
Selected Properties of Chlorophenolsa
|
Chlorophenols
|
Structure
|
MV
b
(cm
3
/mol)
|
C
s
(g/L)
|
LogK
ow
|
pK
a
|
| 2-chlorophenol |
|
99.8 |
2.40 |
2.220 |
8.50 |
| 4-chlorophenol |
|
99.8 |
2.10 |
2.418 |
9.47 |
| 2,4-dichlorophenol |
|
111.7 |
0.47 |
3.095 |
8.05 |
| 2,6-dichlorophenol |
|
111.7 |
0.52 |
2.896 |
7.02 |
| 2,4,5-trichlorophenol |
|
123.7 |
0.085 |
3.835 |
7.10 |
| 2,4,6-trichlorophenol |
|
123.7 |
0.091 |
3.768 |
6.59 |
Abbreviation: MV,molar volume.
aProperties of chlorophenols were culled from (23) and obtained from SciFinder https://scifinder.cas.org.
The recalcitrant nature of CPs makes them discharge into water bodies as a significant source of pollution. Basically, the accurate data are yet to be known, showing the exact amount of CPs discharged into the environment from various processes. Some of the processes applied for treating wastewater contaminated with CPs include adsorption (9-17), catalytic wet oxidation (18), biodegradation (19), aerobic granular sludge technology (20), anaerobic processes (21), and electrochemical degradation (22). However, Das et al (24) believed that the effectiveness of these technologies in fixing water pollution is questionable with their major threats and drawbacks (Fig. 1).
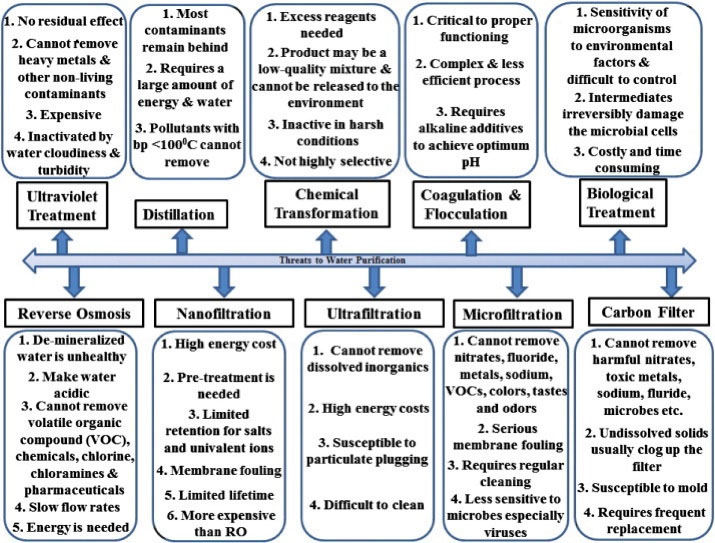
Fig. 1.
Some Major Drawbacks Associated With Conventional Water Purification Systems.
.
Some Major Drawbacks Associated With Conventional Water Purification Systems.
A recently published review article by Garba et al (16) provided detailed information on CPs, their sources into the environment, classification, and toxicity, and various wastewater treatment methods for their removal. In addition, they described the characteristics of CP adsorption by various adsorbents while not including isotherm and kinetic models. Thus, the objective of this work was to show the relevance of isotherm and kinetic models in studying CP adsorption onto various adsorbents.
2. Adsorption Isotherm Models
Adsorption process is globally proven as the most efficient among various water treatment technologies (25,26) since it possesses very fast kinetics, can be easily handled, and is able to remove high amounts of pollutants (27-29). Establishing the most suitable adsorption system for equilibrium data is very crucial for exploring novel adsorbents for an adsorption process (30) which should be reliable in predicting the correlation between adsorption parameters and quantitatively comparing the behavior of adsorbents for systems (31,32). An equilibrium relationship, generally known as adsorption isotherms, is the most suitable system since it perfectly describes the interaction between pollutants and the adsorbent materials, thus becoming very vital in optimizing the mechanisms of adsorption processes, expressing the surface properties and the adsorbents capabilities, and designing the adsorption systems effectively (33,34).
Isotherms are used in studying and explaining the whole adsorption and desorption processes. Further, they are considered as functions that relate the adsorbate amount on the adsorbent with its concentration in case of liquid and its pressure if gaseous at a fixed temperature. Generally, adsorption isotherms comprise invaluable curves that depict how the phenomenon of a substance is governed. Such phenomena occur at a fixed pH and temperature where the retention or mobility of such substances moves from aqueous porous media/environments to a solid-phase (35,36). Adsorption equilibrium is the ratio of the amount of substance adsorbed with that remaining in the solution. This is confirmed when the time, in which an adsorbate containing phase is in contact with the adsorbent, is sufficient for the adsorbate concentration in the bulk solution to be in a dynamic balance with the interface concentration (37,38). A graphical representation of the solid-phase against its residual concentration usually depicts the mathematical correlation, which plays a key role in the modeling analysis, the direction of operational design, and the applicable practices of adsorption systems (39). A better perception of the adsorption mechanism is provided by the adsorbent degree of affinity, along with the underlying thermodynamic and their surface properties (40). Over the years, several researchers have reported various equilibrium isotherm models from three different approaches (41). The first reported approach was the kinetic consideration where adsorption equilibrium was assumed to be a state in which both rates of adsorption and desorption are equal (42). Thermodynamic consideration, where a basis for the derivation of various isotherm models was provided in multiple forms, is the reported second approach (43,44), The third approach was called a potential theory which normally conveys the main idea in generating the characteristic curves (45). However, isotherm derivation from more than one approach is the most fascinating trend in the modeling process, thus leading to the disparity in the physical interpretation of model parameters (46). Table 2 presents various isotherm models based on distinctive assumptions. The assumption with respect to the Langmuir isotherm is monolayer adsorption having a homogeneous surface with a definite number of adsorption sites. On the contrary, the Freundlich model is the most suited model for heterogeneous surfaces and can be applied to multilayer adsorption. In depicting the performance of adsorbents, Langmuir and Freundlich are the most frequently used models for that purpose. Tempkin isotherm assumed that a decrease in adsorption heat is linear. Furthermore, Dubinin-Radushkevich isotherm is commonly engaged in determining whether the adsorption mechanism is chemical or physical with a heterogeneous surface hosting free energy. Similarly, the spontaneous and feasibility nature of the adsorption process is regarded as the major assumption associated with Flory-Huggins isotherm. Another important assumption related to Hill isotherm is how the binding sites of a ligand in a macromolecule can influence other binding sites within the same macromolecule. All the aforementioned isotherms are categorized as two-parameter isotherms. The most popular and frequently used three-parameter isotherms are those in the Redlich-Peterson isotherm model. It combines the Langmuir and Freundlich models that can be used over a vast range of concentrations and in both heterogeneous and homogeneous systems. Other three-parameter isotherms include Sips, Koble-Corrigan, Toth, Radke-Prausnitz, and Khan isotherm (47,60).
Table 2.
Adsorption Isotherm Models (
47)
|
Isotherm models
|
Linear form
|
Nonlinear form
|
Plot
|
Reference
|
| Langmuir |
|
|
|
(42) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Freundlich |
|
|
|
(48) |
| Tempkin |
|
|
|
(49) |
| Dubinin-Radushkevich |
|
|
|
(50) |
| Flory-Huggins |
|
|
|
(51) |
| Hill |
|
|
|
(52) |
| Redlich-Peterson |
|
|
|
(53) |
| Sips |
|
|
|
(54) |
| Toth |
|
|
|
(55) |
| Koble-Corrigan |
|
|
-
|
(56) |
| Polanyi-Manes |
|
|
-
|
(57) |
| Khan |
- |
|
-
|
(58) |
| Radke-Prausnitz |
- |
|
- |
(59) |
3. Error Functions
During the analysis of adsorption experimental data, linear and non-linear isotherm models were used to elaborate on the suitability or best fitting of a model(s) to the process (61). This was aimed toward comparing the experimental data and the predicted isotherm owning to obtain a good understanding of the equilibrium state relationship between adsorbent and adsorbate. Due to linear regression models of popularity and simplicity, many researchers prefer to describe the nature of the adsorption isotherm model and select the optimum model of the process having the minimal error between the experimental data and the predicted isotherm (62-64). However, in some studies, researchers emphasized that the use of nonlinear regression models in analyzing the adsorption equilibrium is more accurate in predicting the optimum isotherm for the process (31). Nonetheless, other researchers believed that both linear and nonlinear regression models are suitable for the selection of best-fitted adsorption equilibrium isotherm depending on the manner of application and magnitude of error (61). Moreover, many error functions are employed in model selection in order to determine the nature of error which still relies on the definition of the intended function. This helps in ascertaining those assertions (64-67).
Rahim and Garba compared the validity of five different linear Langmuir isotherm models by incorporating χ2 since R2 is no longer reliable when it comes to justifying the basis for selecting the most suitable model of adsorption because it only signifies the fit between the linear forms of isotherm equations and experimental data. Additionally, low χ2 values signify the best fit and describe the suitability between the predicted and experimental values of the adsorption capacity (68).
In the present study, several mathematically rigorous error functions were applied in addition to the Chi-square, including hybrid fractional error function, the sum of squares errors, the sum of normalized errors, Spearman’s correlation coefficient, average relative error, the coefficient of non-determination, and the sum of absolute error Marquardt’s percent standard deviation (Table 3). This was done to drastically address and confront the inherent bias resulting from the transformation that leads toward a diverse form of parameter estimation errors and fits distortion (69).
Table 3.
Lists of Error Functions (
47)
|
Error Function
|
Definition
|
Abbreviation
|
Reference
|
| Nonlinear Chi-square test |
|
χ2 |
(70) |
| The coefficient of determination |
|
R2 |
(70) |
| Sum squares errors |
|
ERRSQ/SSE |
(71) |
| The hybrid fractional error function |
|
HYBRID |
(72) |
| Average relative error |
|
ARE |
(73) |
| Sum of absolute error |
|
EABS |
(74) |
| Marquardt’s percent standard deviation |
|
MPSD |
(75) |
| Spearman’s correlation coefficient |
|
rs |
(70) |
| Coefficient of non-determination |
−
|
K2 |
(71) |
| Sum of normalized errors |
−
|
SNE |
(76) |
Note. n: Adsorption intensity; qe, calc: Calculated adsorbate concentration at equilibrium (mg/g); qe, meas: Measured adsorbate concentration at equilibrium (mg/g).
Recently, linear regression has been the most feasible tool among the other instruments for defining the best-fitting relationships (71). In addition, it mathematically analyzes the adsorption systems, quantifies the adsorbates distribution (77), and verifies the consistency and theoretical assumptions of an isotherm model (70). During the development of computer technology in the 1980s, the progression of nonlinear isotherm modeling was broadly facilitated and motivated to be in line with the developing technology (70). In contrast to the model linearization, nonlinear regression usually involves the maximization or minimization of error distribution between the experimental data and the predicted isotherm based on its convergence criteria (69).
4. Reported Isotherm Models for Chlorophenol Adsorption
The reported adsorption isotherms of some CPs onto different adsorbents are summarized in Table 4.
Table 4.
Adsorption Isotherm Models of Chlorophenols Onto Different Adsorbents
|
Adsorbate
|
Adsorbent
|
Isotherm model
|
Reference
|
| 4-Chloroguaiacol |
Oil palm shell activated carbon |
Langmuir |
(78) |
| 4-Chloroguaiacol |
Prosopis africana seed hull activated carbon |
Langmuir |
(68) |
| 2,4,6-Richlorophenol |
Loosestrife activated carbon |
Temkin |
(79) |
| 2,4,6-Trichlorophenol |
Cattail fibre-based activated carbon |
Freundlich |
(80) |
| 2,4,6-Trichlorophenol |
Coconut husk-based activated carbon |
Langmuir |
(81) |
| 2,4,6-Trichlorophenol |
Copper (II)-halloysite nanotubes |
Freundlich |
(82) |
| 2,4,6-Trichlorophenol |
Activated carbon from coconut shell |
Freundlich |
(83) |
| 2,4-Dichlorophenol |
Cattail fibre-based activated carbon |
Freundlich |
(80) |
| 2,4-Dichlorophenol |
Organo clays |
Langmuir |
(84) |
| 2,4-Dichlorophenol |
Prosopis africana seed hull activated carbon |
Langmuir |
(12) |
| 2,4-Dichlorophenol |
Single-walled carbon nanotubes |
Langmuir |
(23) |
| 2,4-Dichlorophenol |
Single-walled carbon nanotubes |
Polanyi-Manes |
(23) |
| 2,6-Dichlorophenol |
Modified plantain peel |
Freundlich |
(85) |
| 4-Chlorophenol |
Rattan sawdust based activated carbon |
Langmuir |
(86) |
| 4-Chlorophenol |
Chemically modified chitosan |
Freundlich |
(87) |
| 4-Chlorophenol |
Porous carbon from coconut spathe |
Langmuir |
(88) |
| 4-Chlorophenol |
Nanosized activated carbon |
Freundlich |
(9) |
| 4-Chlorophenol |
Carbon nanofibers |
Langmuir |
(13) |
| 3-Chlorophenol |
Purified multiwalled carbon nanotubes |
Langmuir |
(89) |
| 3-Chlorophenol |
Rice-straw-based carbon |
Langmuir |
(90) |
| 2-Chlorophenol |
Single-walled carbon nanotubes |
Polanyi-Manes |
(23) |
The functionalization of single-walled carbon nanotubes (SWCNTs) was reported by Ding et al (23), which was applied for CP adsorption from aqueous solution. They indicated that three models well fit the isotherms with the presence of grooves, an external surface, and interstitial channel adsorption sites in the closed-ended bundles of the SWCNT (91). The researchers further revealed that the SWCNT interstitial channels are extremely tiny for the adsorbate molecules to fit into, thus proposing the major adsorption sites to rely on grooves and external surface area (92). As a result, surface adsorption dominates the adsorption process. Considering that the Langmuir model and Polanyi-Manes models were both derived from surface adsorption (57,93) and the Freundlich model was a special form of Polanyi-Manes model (94), it was reasonable that the adsorption data were well fitted to those three models.
In another development, Ren et al (80) reported Freundlich as the most suitable isotherm model in describing the adsorption of 2,4-dichlorophenol and 2,4,6-trichlorophenol onto cattail fibre-based activated carbon. Their revelation hinted at the uptake of both 2,4-dichlorophenol and 2,4,6-trichlorophenos was multimolecular layer adsorption with interactions between the adsorbed molecules, and the surface of the cattail fibre-based activated carbon was relatively heterogeneous. Additionally, Garba and Rahim (12) studied the adsorptive removal of para-chlorophenol and 2,4-dichlorophenol by employing the activated carbon from Prosopis africana seed hulls as the adsorbent. They carried out their experiments at three different temperatures by fitting the experimental data into Langmuir, Freundlich, and Temkin models and reported that the R2 of the three models was higher than 0.90 for all studied temperatures. However, the applicability of the Langmuir isotherm model became more pronounced compared to the other two isotherms when they applied the chi-square values. The fitness of the Langmuir model to the adsorption process connotes that the two CP molecules from bulk solution were adsorbed on specific monolayer which is homogeneous in nature.
As shown in Table 4, Langmuir and Freundlich were the most important isotherm models in fitting the CP adsorption data with the nature of the adsorbent, as well as the type of CPs playing a significant role in determining whether the process is monolayer or multilayer on a homogeneous or heterogeneous surface, respectively.
5. Adsorption Kinetic Studies
Elovich equation and Largergren pseudo-first and-second order (1 & 2) models are the most popular and widely used models for kinetic study. This facilitates the understanding of the dynamics of an adsorption process.
5.1. Pseudo-first Order Model
The adsorption rate constant is ascertained from the pseudo-first order equation given by (95) as:
(1)
(1)
Where qe and qe are the amount of CPs adsorbed onto the adsorbents (mg. g-1) at equilibrium and at time t, respectively, while k1 is the pseudo-first order rate constant (h−1).
5.2. Pseudo-second Order (1 & 2)
The pseudo-second order equation is expressed in two linear forms (95):
(3)
where k2 (g. mg.-1 h-1) is the rate constant of the second-order adsorption.
5.3. Elovich Kinetic Model
The primary proposed Elovich equation was to describe the kinetics of gases and chemisorptions on the solids. The model shows a variation in the energy of chemisorptions, which is ascribed to a change in surface coverage or a continuous and specific range of site reactivity. Its linear equation is expressed as (79):
The term (
) stands for the number of attainable adsorption sites whereas (
)ln(ab) denotes the quantity of adsorption lnt = 0. (79).
The examples of the adsorption kinetics of chlorophenols onto different adsorbents are summarized in Table 5.
Table 5.
Adsorption Kinetics of Chlorophenols Onto Different Adsorbents
|
Adsorbate
|
Adsorbent
|
Kinetic model
|
Reference
|
| 4-Chloroguaiacol |
Oil palm shell activated carbon |
Pseudo-second order |
(78) |
| 4-Chloroguaiacol |
Prosopis africana seed hull activated carbon |
Pseudo-second order |
(68) |
| 4-Chloro-2-methoxyphenol |
Oil palm shell activated carbon |
Pseudo-second order |
(2) |
| 2,4,6-Trichlorophenol |
Loosestrife activated carbon |
Pseudo-second order |
(79) |
| 2,4,6-Trichlorophenol |
Copper (II)–halloysite |
Pseudo-second order |
(82) |
| 2,4,6-Trichlorophenol |
Chemically modified chitosan |
Pseudo-second order |
(87) |
| 2,4,6-Trichlorophenol |
Cattail fibre-based activated carbon |
Pseudo-second order |
(80) |
| 2,4-Dichlorophenol |
Organo clays |
Pseudo-second order |
(84) |
| 2,4-Dichlorophenol |
Polyimide (PI)-based carbon nanofibers |
Pseudo-second order |
(99) |
| 2,4-Dichlorophenol |
Chemically modified chitosan |
Pseudo-second order |
(87) |
| 2,6-Dichlorophenol |
Modified plantain peel |
Pseudo-second order |
(85) |
| 4-Chlorophenol |
Rattan sawdust based activated carbon |
Pseudo-second order |
(86) |
| 4-Chlorophenol |
Chemically modified chitosan |
Pseudo-second order |
(87) |
6. Adsorption Thermodynamics
The adsorption thermodynamics of the CPs are studied to verify the adsorption process spontaneity. The most popular studied thermodynamic parameters are entropy change (∆S), Gibb’s free energy change (∆G), and enthalpy change (∆H). The evaluation of the thermodynamic parameters is carried out using Van’t Hoff equation which is expressed as:
where KD, T, and R represent the distribution coefficient, the absolute temperature (K), and the universal gas constant (8.314 J mol-1 K-1), respectively. A linear graphical representation is obtained when ln KD is plotted against 1/T deriving ∆H and ∆S from the slope and intercept, respectively. In this study, ∆G was evaluated from the Gibbs-Helmholtz relation (96-98):
Physisorption was revealed to describe the adsorption of phenolic compounds on a water-compatible hypercrosslinked polymeric resin (101), as well as from agro-based derived adsorbents (102). On the other hand, the adsorption of pure phenolics onto activated carbons and polymeric resins (103), modified macroalga (104), as well as viable fungal biomass (105) was described as exothermic based on the obtained ∆H values. Endothermic processes were also reported for the adsorption of pure phenols onto waste leached residue, from manganese production (106), and the vegetal cords (107). The same process was also reported for mixed standard phenols onto activated carbon from the olive husk (108).
7. Conclusion and Future Outlook
In this review, the adsorption process was reported for its attributes in effectiveness, simplicity in design and applicability, fast kinetics, and its universal nature for the removal of CPs from wastewater among the other wastewater treatment methods. A number of adsorbents such as agricultural wastes, active carbons, industrial by-products, biosorbents, and nanomaterials were used at various conditions for the decontamination of wastewater containing CPs. However, in the adsorption system designing, researchers have developed and revealed linear regression as the most preferable option in the last few decades despite indications from recent investigations signaling an increasing discrepancy and shortcoming of the model, which propagates toward a different outcome. Despite the above-mentioned explanations, linearization remains the most trusted option in the literature and statistics prove its application in over 95% of the liquid-phase adsorption systems. Hence, the next real challenge in the adsorption field is the identification and clarification of both isotherm models in various adsorption systems.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
References
- Ma X, Liu Y, Li X, Xu J, Gu G, Xia C. Water: the most effective solvent for liquid-phase hydrodechlorination of chlorophenols over Raney Ni catalyst. Appl Catal B Environ 2015; 165:351-9. doi: 10.1016/j.apcatb.2014.10.035 [Crossref] [ Google Scholar]
- Hamad BK, Noor AM, Rahim AA. Removal of 4-chloro-2-methoxyphenol from aqueous solution by adsorption to oil palm shell activated carbon activated with K2CO3. J Phys Sci 2011; 22(1):39-55. [ Google Scholar]
- Galadima A, Garba ZN, Leke L, Almustapha MN, Adam IK. Domestic Water Pollution among Local Communities in Nigeria ----Causes and Consequences. European Journal of Scientific Research 2011; 52(4):592-603. [ Google Scholar]
- Fan C, Li N, Cao X. Determination of chlorophenols in honey samples using in-situ ionic liquid-dispersive liquid-liquid microextraction as a pretreatment method followed by high-performance liquid chromatography. Food Chem 2015; 174:446-51. doi: 10.1016/j.foodchem.2014.11.050 [Crossref] [ Google Scholar]
- Sarnaik S, Kanekar P. Bioremediation of colour of methyl violet and phenol from a dye-industry waste effluent using Pseudomonas spp isolated from factory soil. J Appl Bacteriol 1995; 79(4):459-69. doi: 10.1111/j.1365-2672.1995.tb03162.x [Crossref] [ Google Scholar]
- Armenante PM, Kafkewitz D, Lewandowski GA, Jou C-J. Anaerobic–aerobic treatment of halogenated phenolic compounds. Water Res 1999; 33(3):681-92. doi: 10.1016/S0043-1354(98)00255-3 [Crossref] [ Google Scholar]
- Tan IA, Ahmad AL, Hameed BH. Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J Hazard Mater 2009; 164(2-3):473-82. doi: 10.1016/j.jhazmat.2008.08.025 [Crossref] [ Google Scholar]
- Gupta VK, Carrott PJM, Ribeiro Carrott MML, Suhas Suhas. Low-cost adsorbents: growing approach to wastewater treatment--a review. Crit Rev Environ Sci Technol 2009; 39(10):783-842. doi: 10.1080/10643380801977610 [Crossref] [ Google Scholar]
- Chen C, Geng X, Huang W. Adsorption of 4-chlorophenol and aniline by nanosized activated carbons. Chem Eng J 2017; 327:941-52. doi: 10.1016/j.cej.2017.06.183 [Crossref] [ Google Scholar]
- Garba ZN, Rahim AA. Adsorption of 4-chlorophenol onto optimum activated carbon from an agricultural waste. Int J Sci Res 2015; 4(5):1931-6. [ Google Scholar]
- Garba ZN, Rahim AA. Process optimization of K2C2O4-activated carbon from Prosopis africana seed hulls using response surface methodology. J Anal Appl Pyrolysis 2014; 107:306-12. doi: 10.1016/j.jaap.2014.03.016 [Crossref] [ Google Scholar]
- Garba ZN, Rahim AA. Evaluation of optimal activated carbon from an agricultural waste for the removal of para-chlorophenol and 2,4-dichlorophenol. Process Saf Environ Prot 2016; 102:54-63. doi: 10.1016/j.psep.2016.02.006 [Crossref] [ Google Scholar]
- Madannejad S, Rashidi A, Sadeghhassani S, Shemirani F, Ghasemy E. Removal of 4-chlorophenol from water using different carbon nanostructures: a comparison study. J Mol Liq 2018; 249:877-85. doi: 10.1016/j.molliq.2017.11.089 [Crossref] [ Google Scholar]
- Adetokun AA, Uba S, Garba ZN. Optimization of adsorption of metal ions from a ternary aqueous solution with activated carbon from Acacia senegal (L.) Willd pods using Central Composite Design. J King Saud Univ Sci. 2018. 10.1016/j.jksus.2018.12.007 .
- Garba ZN, Hussin MH, Galadima A, Lawan I. Potentials of Canarium schweinfurthii seed shell as a novel precursor for CH3COOK activated carbon: statistical optimization, equilibrium and kinetic studies. Appl Water Sci 2019; 9(2):31. doi: 10.1007/s13201-019-0907-y [Crossref] [ Google Scholar]
- Garba ZN, Zhou W, Lawan I, Xiao W, Zhang M, Wang L. An overview of chlorophenols as contaminants and their removal from wastewater by adsorption: a review. J Environ Manage 2019; 241:59-75. doi: 10.1016/j.jenvman.2019.04.004 [Crossref] [ Google Scholar]
- Garcia-Muñoz P, Lefevre C, Robert D, Keller N. Ti-substituted LaFeO3 perovskite as photoassisted CWPO catalyst for water treatment. Appl Catal B Environ 2019; 248:120-8. doi: 10.1016/j.apcatb.2019.02.030 [Crossref] [ Google Scholar]
- Chaliha S, Bhattacharyya KG. Catalytic wet oxidation of 2-chlorophenol, 2,4-dichlorophenol and 2,4,6-trichlorophenol in water with Mn(II)-MCM41. Chem Eng J 2008; 139(3):575-88. doi: 10.1016/j.cej.2007.09.006 [Crossref] [ Google Scholar]
- Steinle P, Thalmann P, Höhener P, Hanselmann KW, Stucki G. Effect of environmental factors on the degradation of 2, 6-dichlorophenol in soil. Environ Sci Technol 2000; 34(5):771-5. doi: 10.1021/es990587l [Crossref] [ Google Scholar]
- Bengtsson S, de Blois M, Wilén B-M, Gustavsson D. Treatment of municipal wastewater with aerobic granular sludge. Crit Rev Environ Sci Technol 2018; 48(2):119-66. doi: 10.1080/10643389.2018.1439653 [Crossref] [ Google Scholar]
- Mai DT, Kunacheva C, Stuckey DC. A review of posttreatment technologies for anaerobic effluents for discharge and recycling of wastewater. Crit Rev Environ Sci Technol 2018; 48(2):167-209. doi: 10.1080/10643389.2018.1443667 [Crossref] [ Google Scholar]
- Lim JW, Lim PE, Seng CE, Adnan R. Simultaneous 4-chlorophenol and nitrogen removal in moving bed sequencing batch reactors packed with polyurethane foam cubes of various sizes. Bioresour Technol 2013; 129:485-94. doi: 10.1016/j.biortech.2012.11.111 [Crossref] [ Google Scholar]
- Ding H, Li X, Wang J, Zhang X, Chen C. Adsorption of chlorophenols from aqueous solutions by pristine and surface functionalized single-walled carbon nanotubes. J Environ Sci (China) 2016; 43:187-98. doi: 10.1016/j.jes.2015.09.004 [Crossref] [ Google Scholar]
- Das R, Ali ME, Hamid SBA, Ramakrishna S, Chowdhury ZZ. Carbon nanotube membranes for water purification: a bright future in water desalination. Desalination 2014; 336:97-109. doi: 10.1016/j.desal.2013.12.026 [Crossref] [ Google Scholar]
- Giraldo L, Moreno-Piraján JC. Study of adsorption of phenol on activated carbons obtained from eggshells. J Anal Appl Pyrolysis 2014; 106:41-7. doi: 10.1016/j.jaap.2013.12.007 [Crossref] [ Google Scholar]
- Foroughi-Dahr M, Abolghasemi H, Esmaili M, Shojamoradi A, Fatoorehchi H. Adsorption characteristics of Congo red from aqueous solution onto tea waste. Chem Eng Commun 2015; 202(2):181-93. doi: 10.1080/00986445.2013.836633 [Crossref] [ Google Scholar]
- Lladó J, Lao-Luque C, Ruiz B, Fuente E, Solé-Sardans M, Dorado AD. Role of activated carbon properties in atrazine and paracetamol adsorption equilibrium and kinetics. Process Saf Environ Prot 2015; 95:51-9. doi: 10.1016/j.psep.2015.02.013 [Crossref] [ Google Scholar]
- Lee LY, Chin DZB, Lee XJ, Chemmangattuvalappil N, Gan S. Evaluation of Abelmoschus esculentus (lady’s finger) seed as a novel biosorbent for the removal of Acid Blue 113 dye from aqueous solutions. Process Saf Environ Prot 2015; 94:329-38. doi: 10.1016/j.psep.2014.08.004 [Crossref] [ Google Scholar]
- Xu Z, Zhang Q, Fang HHP. Applications of porous resin sorbents in industrial wastewater treatment and resource recovery. Crit Rev Environ Sci Technol 2003; 33(4):363-89. doi: 10.1080/10643380390249512 [Crossref] [ Google Scholar]
- Srivastava VC, Swamy MM, Mall ID, Prasad B, Mishra IM. Adsorptive removal of phenol by bagasse fly ash and activated carbon: equilibrium, kinetics and thermodynamics. Colloids Surf A Physicochem Eng Asp 2006; 272(1):89-104. doi: 10.1016/j.colsurfa.2005.07.016 [Crossref] [ Google Scholar]
- Gimbert F, Morin-Crini N, Renault F, Badot PM, Crini G. Adsorption isotherm models for dye removal by cationized starch-based material in a single component system: error analysis. J Hazard Mater 2008; 157(1):34-46. doi: 10.1016/j.jhazmat.2007.12.072 [Crossref] [ Google Scholar]
- Ho YS, Porter JF, McKay G. Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: copper, nickel and lead single component systems. Water Air Soil Pollut 2002; 141(1):1-33. doi: 10.1023/a:1021304828010 [Crossref] [ Google Scholar]
- El-Khaiary MI. Least-squares regression of adsorption equilibrium data: comparing the options. J Hazard Mater 2008; 158(1):73-87. doi: 10.1016/j.jhazmat.2008.01.052 [Crossref] [ Google Scholar]
- Thompson G, Swain J, Kay M, Forster CF. The treatment of pulp and paper mill effluent: a review. Bioresour Technol 2001; 77(3):275-86. doi: 10.1016/s0960-8524(00)00060-2 [Crossref] [ Google Scholar]
- Allen SJ, McKay G, Porter JF. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J Colloid Interface Sci 2004; 280(2):322-33. doi: 10.1016/j.jcis.2004.08.078 [Crossref] [ Google Scholar]
- Limousin G, Gaudet JP, Charlet L, Szenknect S, Barthès V, Krimissa M. Sorption isotherms: a review on physical bases, modeling and measurement. Appl Geochem 2007; 22(2):249-75. doi: 10.1016/j.apgeochem.2006.09.010 [Crossref] [ Google Scholar]
- Ghiaci M, Abbaspur A, Kia R, Seyedeyn-Azad F. Equilibrium isotherm studies for the sorption of benzene, toluene, and phenol onto organo-zeolites and as-synthesized MCM-41. Sep Purif Technol 2004; 40(3):217-29. doi: 10.1016/j.seppur.2004.03.001 [Crossref] [ Google Scholar]
- Vasanth Kumar K, Sivanesan S. Sorption isotherm for safranin onto rice husk: comparison of linear and non-linear methods. Dyes Pigm 2007; 72(1):130-3. doi: 10.1016/j.dyepig.2005.07.020 [Crossref] [ Google Scholar]
- Ncibi MC. Applicability of some statistical tools to predict optimum adsorption isotherm after linear and non-linear regression analysis. J Hazard Mater 2008; 153(1-2):207-12. doi: 10.1016/j.jhazmat.2007.08.038 [Crossref] [ Google Scholar]
- Bulut E, Özacar M, Şengil İA. Adsorption of malachite green onto bentonite: equilibrium and kinetic studies and process design. Microporous Mesoporous Mater 2008; 115(3):234-46. doi: 10.1016/j.micromeso.2008.01.039 [Crossref] [ Google Scholar]
- Malek A, Farooq S. Comparison of isotherm models for hydrocarbon adsorption on activated carbon. AIChE J 1996; 42(11):3191-201. doi: 10.1002/aic.690421120 [Crossref] [ Google Scholar]
- Langmuir I. The constitution and fundamental properties of solids and liquids. J Franklin Inst 1917; 183(1):102-5. doi: 10.1016/S0016-0032(17)90938-X [Crossref] [ Google Scholar]
- de Boer JH. The Dynamical Character of Adsorption. 2nd ed. London: Oxford University Press; 1968.
- Myers AL, Prausnitz JM. Thermodynamics of mixed-gas adsorption. AIChE J 1965; 11(1):121-7. doi: 10.1002/aic.690110125 [Crossref] [ Google Scholar]
- Dubinin MM. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem Rev 1960; 60(2):235-41. doi: 10.1021/cr60204a006 [Crossref] [ Google Scholar]
- Ruthven DM. Principles of Adsorption and Adsorption Processes. New York: Wiley; 1984.
- Foo KY, Hameed BH. Insights into the modeling of adsorption isotherm systems. Chem Eng J 2010; 156(1):2-10. doi: 10.1016/j.cej.2009.09.013 [Crossref] [ Google Scholar]
- Freundlich HMF. Over the adsorption in solution. J Phys Chem 1906; 57:385-470. [ Google Scholar]
- Temkin MI, Pyzhev V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Physicochimica URSS 1940; 12:327-56. [ Google Scholar]
- Dubinin MM, Radushkevich LV. The equation of the characteristic curve of the activated charcoal. Proceedings of the USSR Academy of Sciences, Physical Chemistry Section; 1947.
- Jnr MH, Spiff AI. Equilibrium sorption study of Al3+, Co2+ and Ag+ in aqueous solutions by fluted pumpkin (Telfairia occidentalis HOOK f) waste biomass. Acta Chim Slov 2005; 52:174-81. [ Google Scholar]
- Hill AV. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol 1910; 40:4-7. [ Google Scholar]
- Redlich O, Peterson DL. A useful adsorption isotherm. J Phys Chem 1959; 63(6):1024. doi: 10.1021/j150576a611 [Crossref] [ Google Scholar]
- Sips R. Combined form of Langmuir and Freundlich equations. J Chem Phys 1948; 16(5):490-5. [ Google Scholar]
- Toth J. State equations of the solid gas interface layer. Acta Chim Acad Sci Hung 1971; 69:311-7. [ Google Scholar]
- Koble RA, Corrigan TE. Adsorption isotherms for pure hydrocarbons. Ind Eng Chem 1952; 44(2):383-7. doi: 10.1021/ie50506a049 [Crossref] [ Google Scholar]
- Yang K, Xing B. Adsorption of organic compounds by carbon nanomaterials in aqueous phase: polanyi theory and its application. Chem Rev 2010; 110(10):5989-6008. doi: 10.1021/cr100059s [Crossref] [ Google Scholar]
- Khan AR, Ataullah R, Al-Haddad A. Equilibrium adsorption studies of some aromatic pollutants from dilute aqueous solutions on activated carbon at different temperatures. J Colloid Interface Sci 1997; 194(1):154-65. doi: 10.1006/jcis.1997.5041 [Crossref] [ Google Scholar]
- Vijayaraghavan K, Padmesh TVN, Palanivelu K, Velan M. Biosorption of nickel(II) ions onto Sargassum wightii: application of two-parameter and three-parameter isotherm models. J Hazard Mater 2006; 133(1-3):304-8. doi: 10.1016/j.jhazmat.2005.10.016 [Crossref] [ Google Scholar]
- Singh NB, Nagpal G, Agrawal S, Rachna Rachna. Water purification by using adsorbents: a review. Environ Technol Innov 2018; 11:187-240. doi: 10.1016/j.eti.2018.05.006 [Crossref] [ Google Scholar]
- Ofomaja AE, Ho YS. Kinetic biosorption study of cadmium onto coconut copra meal as biosorbent. Int J Environ Pollut 2008; 34(1-4):466-76. doi: 10.1504/IJEP.2008.020810 [Crossref] [ Google Scholar]
- Ayoob S, Gupta AK. Insights into isotherm making in the sorptive removal of fluoride from drinking water. J Hazard Mater 2008; 152(3):976-85. doi: 10.1016/j.jhazmat.2007.07.072 [Crossref] [ Google Scholar]
- He J, Hong S, Zhang L, Gan F, Ho YS. Equilibrium and thermodynamic parameters of adsorption of methylene blue onto rectorite. Fresenius Environ Bull 2010; 19(11):2651-6. [ Google Scholar]
- Ho YS. Isotherms for the sorption of lead onto peat: comparison of linear and non-linear methods. Pol J Environ Stud 2006; 15(1):81-6. [ Google Scholar]
- Alihosseini A, Taghikhani V, Safekordi AA, Bastani D. Equilibrium sorption of crude oil by expanded perlite using different adsorption isotherms at 29815 k. Int J Environ Sci Technol 2010; 7(3):591-8. doi: 10.1007/bf03326168 [Crossref] [ Google Scholar]
- Brdar M, Takači A, Šćiban M, Rakić D. Isotherms for the adsorption of Cu (II) onto lignin: comparison of linear and non-linear methods. Hem Ind 2012; 66(4):497-503. [ Google Scholar]
- Kumar KV, Porkodi K, Rocha F. Comparison of various error functions in predicting the optimum isotherm by linear and non-linear regression analysis for the sorption of basic red 9 by activated carbon. J Hazard Mater 2008; 150(1):158-65. doi: 10.1016/j.jhazmat.2007.09.020 [Crossref] [ Google Scholar]
- Rahim AA, Garba ZN. Efficient adsorption of 4-chloroguiacol from aqueous solution using optimal activated carbon: equilibrium isotherms and kinetics modeling. Journal of the Association of Arab Universities for Basic and Applied Sciences 2016; 21:17-23. doi: 10.1016/j.jaubas.2015.09.001 [Crossref] [ Google Scholar]
- Kumar KV, Porkodi K, Rocha F. Isotherms and thermodynamics by linear and non-linear regression analysis for the sorption of methylene blue onto activated carbon: comparison of various error functions. J Hazard Mater 2008; 151(2-3):794-804. doi: 10.1016/j.jhazmat.2007.06.056 [Crossref] [ Google Scholar]
- Boulinguiez B, Le Cloirec P, Wolbert D. Revisiting the determination of Langmuir parameters--application to tetrahydrothiophene adsorption onto activated carbon. Langmuir 2008; 24(13):6420-4. doi: 10.1021/la800725s [Crossref] [ Google Scholar]
- Kumar KV, Sivanesan S. Pseudo second order kinetics and pseudo isotherms for malachite green onto activated carbon: comparison of linear and non-linear regression methods. J Hazard Mater 2006; 136(3):721-6. doi: 10.1016/j.jhazmat.2006.01.003 [Crossref] [ Google Scholar]
- Ng JC, Cheung WH, McKay G. Equilibrium studies of the sorption of Cu(II) ions onto chitosan. J Colloid Interface Sci 2002; 255(1):64-74. doi: 10.1006/jcis.2002.8664 [Crossref] [ Google Scholar]
- Kapoor A, Yang RT. Correlation of equilibrium adsorption data of condensable vapours on porous adsorbents. Gas Sep Purif 1989; 3:187-92. [ Google Scholar]
- Ng JC, Cheung WH, McKay G. Equilibrium studies for the sorption of lead from effluents using chitosan. Chemosphere 2003; 52(6):1021-30. doi: 10.1016/s0045-6535(03)00223-6 [Crossref] [ Google Scholar]
- Marquardt DW. An algorithm for least-squares estimation of nonlinear parameters. J Soc Ind Appl Math 1963; 11(2):431-41. [ Google Scholar]
- Karadag D, Koc Y, Turan M, Ozturk M. A comparative study of linear and non-linear regression analysis for ammonium exchange by clinoptilolite zeolite. J Hazard Mater 2007; 144(1-2):432-7. doi: 10.1016/j.jhazmat.2006.10.055 [Crossref] [ Google Scholar]
- Lataye DH, Mishra IM, Mall ID. Adsorption of 2-picoline onto bagasse fly ash from aqueous solution. Chem Eng J 2008; 138(1-3):35-46. doi: 10.1016/j.cej.2007.05.043 [Crossref] [ Google Scholar]
- Hamad BK, Noor AM, Rahim AA, Mohd Asri MN. High removal of 4-chloroguaiacol by high surface area of oil palm shell-activated carbon activated with NaOH from aqueous solution. Desalination 2010; 257(1-3):1-7. doi: 10.1016/j.desal.2010.03.007 [Crossref] [ Google Scholar]
- Fan J, Zhang J, Zhang C, Ren L, Shi Q. Adsorption of 2,4,6-trichlorophenol from aqueous solution onto activated carbon derived from loosestrife. Desalination 2011; 267(2-3):139-46. doi: 10.1016/j.desal.2010.09.016 [Crossref] [ Google Scholar]
- Ren L, Zhang J, Li Y, Zhang C. Preparation and evaluation of cattail fiber-based activated carbon for 2,4-dichlorophenol and 2,4,6-trichlorophenol removal. Chem Eng J 2011; 168(2):553-61. doi: 10.1016/j.cej.2011.01.021 [Crossref] [ Google Scholar]
- Hameed BH, Tan IAW, Ahmad AL. Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chem Eng J 2008; 144(2):235-44. doi: 10.1016/j.cej.2008.01.028 [Crossref] [ Google Scholar]
- Zango ZU, Garba ZN, Abu Bakar NHH, Tan WL, Abu Bakar M. Adsorption studies of Cu2+–Hal nanocomposites for the removal of 2,4,6-trichlorophenol. Appl Clay Sci 2016; 132-133:68-78. doi: 10.1016/j.clay.2016.05.016 [Crossref] [ Google Scholar]
- Radhika M, Palanivelu K. Adsorptive removal of chlorophenols from aqueous solution by low cost adsorbent--Kinetics and isotherm analysis. J Hazard Mater 2006; 138(1):116-24. doi: 10.1016/j.jhazmat.2006.05.045 [Crossref] [ Google Scholar]
- Huang L, Zhou Y, Guo X, Chen Z. Simultaneous removal of 2,4-dichlorophenol and Pb(II) from aqueous solution using organoclays: isotherm, kinetics and mechanism. J Ind Eng Chem 2015; 22:280-7. doi: 10.1016/j.jiec.2014.07.021 [Crossref] [ Google Scholar]
- Agarry SE, Owabor CN, Ajani AO. Modified plantain peel as cellulose-based low-cost adsorbent for the removal of 2, 6-dichlorophenol from aqueous solution: adsorption isotherms, kinetic modeling, and thermodynamic studies. Chem Eng Commun 2013; 200(8):1121-47. doi: 10.1080/00986445.2012.740534 [Crossref] [ Google Scholar]
- Hameed BH, Chin LH, Rengaraj S. Adsorption of 4-chlorophenol onto activated carbon prepared from rattan sawdust. Desalination 2008; 225(1-3):185-98. doi: 10.1016/j.desal.2007.04.095 [Crossref] [ Google Scholar]
- Zhou LC, Meng XG, Fu JW, Yang YC, Yang P, Mi C. Highly efficient adsorption of chlorophenols onto chemically modified chitosan. Appl Surf Sci 2014; 292:735-41. doi: 10.1016/j.apsusc.2013.12.041 [Crossref] [ Google Scholar]
- Prashanthakumar TKM, Kumar SKA, Sahoo SK. A quick removal of toxic phenolic compounds using porous carbon prepared from renewable biomass coconut spathe and exploration of new source for porous carbon materials. J Environ Chem Eng 2018; 6(1):1434-42. doi: 10.1016/j.jece.2018.01.051 [Crossref] [ Google Scholar]
- Tóth A, Törőcsik A, Tombácz E, László K. Competitive adsorption of phenol and 3-chlorophenol on purified MWCNTs. J Colloid Interface Sci 2012; 387(1):244-9. doi: 10.1016/j.jcis.2012.07.064 [Crossref] [ Google Scholar]
- Wang SL, Tzou YM, Lu YH, Sheng G. Removal of 3-chlorophenol from water using rice-straw-based carbon. J Hazard Mater 2007; 147(1-2):313-8. doi: 10.1016/j.jhazmat.2007.01.031 [Crossref] [ Google Scholar]
- Ren X, Chen C, Nagatsu M, Wang X. Carbon nanotubes as adsorbents in environmental pollution management: a review. Chem Eng J 2011; 170(2-3):395-410. doi: 10.1016/j.cej.2010.08.045 [Crossref] [ Google Scholar]
- Pan B, Lin DH, Mashayekhi H, Xing BS. Adsorption and hysteresis of bisphenol A and 17 alpha-ethinyl estradiol on carbon nanomaterials. Environ Sci Technol 2008; 42(15):5480-5. doi: 10.1021/es8001184 [Crossref] [ Google Scholar]
- Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 1918; 40(9):1361-403. doi: 10.1021/ja02242a004 [Crossref] [ Google Scholar]
- Yang K, Zhu L, Xing B. Adsorption of polycyclic aromatic hydrocarbons by carbon nanomaterials. Environ Sci Technol 2006; 40(6):1855-61. doi: 10.1021/es052208w [Crossref] [ Google Scholar]
- Kumar M, Tamilarasan R. Modeling studies for the removal of methylene blue from aqueous solution using Acacia fumosa seed shell activated carbon. J Environ Chem Eng 2013; 1(4):1108-16. doi: 10.1016/j.jece.2013.08.027 [Crossref] [ Google Scholar]
- Gupta VK, Mittal A, Krishnan L, Gajbe V. Adsorption kinetics and column operations for the removal and recovery of malachite green from wastewater using bottom ash. Sep Purif Technol 2004; 40(1):87-96. doi: 10.1016/j.seppur.2004.01.008 [Crossref] [ Google Scholar]
- Huang L, Wang M, Shi C, Huang J, Zhang B. Adsorption of tetracycline and ciprofloxacin on activated carbon prepared from lignin with H3PO4 activation. Desalin Water Treat 2014; 52(13-15):2678-87. doi: 10.1080/19443994.2013.833873 [Crossref] [ Google Scholar]
- Soto ML, Moure A, Domínguez H, Parajó JC. Recovery, concentration and purification of phenolic compounds by adsorption: a review. J Food Eng 2011; 105(1):1-27. doi: 10.1016/j.jfoodeng.2011.02.010 [Crossref] [ Google Scholar]
- Zhang Y, Ou H, Liu H, Ke Y, Zhang W, Liao G. Polyimide-based carbon nanofibers: a versatile adsorbent for highly efficient removals of chlorophenols, dyes and antibiotics. Colloids Surf A Physicochem Eng Asp 2018; 537:92-101. doi: 10.1016/j.colsurfa.2017.10.014 [Crossref] [ Google Scholar]
- Li X, Chen S, Fan X, Quan X, Tan F, Zhang Y. Adsorption of ciprofloxacin, bisphenol and 2-chlorophenol on electrospun carbon nanofibers: in comparison with powder activated carbon. J Colloid Interface Sci 2015; 447:120-7. doi: 10.1016/j.jcis.2015.01.042 [Crossref] [ Google Scholar]
- Li A, Zhang Q, Zhang G, Chen J, Fei Z, Liu F. Adsorption of phenolic compounds from aqueous solutions by a water-compatible hypercrosslinked polymeric adsorbent. Chemosphere 2002; 47(9):981-9. doi: 10.1016/S0045-6535(01)00222-3 [Crossref] [ Google Scholar]
- Srihari V, Das A. Comparative studies on adsorptive removal of phenol by three agro-based carbons: equilibrium and isotherm studies. Ecotoxicol Environ Saf 2008; 71(1):274-83. doi: 10.1016/j.ecoenv.2007.08.008 [Crossref] [ Google Scholar]
- Huang J, Huang K, Yan C. Application of an easily water-compatible hypercrosslinked polymeric adsorbent for efficient removal of catechol and resorcinol in aqueous solution. J Hazard Mater 2009; 167(1-3):69-74. doi: 10.1016/j.jhazmat.2008.12.120 [Crossref] [ Google Scholar]
- Aravindhan R, Rao JR, Nair BU. Application of a chemically modified green macro alga as a biosorbent for phenol removal. J Environ Manage 2009; 90(5):1877-83. doi: 10.1016/j.jenvman.2008.12.005 [Crossref] [ Google Scholar]
- Mathialagan T, Viraraghavan T. Biosorption of pentachlorophenol from aqueous solutions by a fungal biomass. Bioresour Technol 2009; 100(2):549-58. doi: 10.1016/j.biortech.2008.06.054 [Crossref] [ Google Scholar]
- Parida KM, Pradhan AC. Removal of phenolic compounds from aqueous solutions by adsorption onto manganese nodule leached residue. J Hazard Mater 2010; 173(1-3):758-64. doi: 10.1016/j.jhazmat.2009.09.003 [Crossref] [ Google Scholar]
- Cherifi H, Hanini S, Bentahar F. Adsorption of phenol from wastewater using vegetal cords as a new adsorbent. Desalination 2009; 244(1):177-87. doi: 10.1016/j.desal.2008.05.022 [Crossref] [ Google Scholar]
- Michailof C, Stavropoulos GG, Panayiotou C. Enhanced adsorption of phenolic compounds, commonly encountered in olive mill wastewaters, on olive husk derived activated carbons. Bioresour Technol 2008; 99(14):6400-8. doi: 10.1016/j.biortech.2007.11.057 [Crossref] [ Google Scholar]