Avicenna J Environ Health Eng. 11(1):55-62.
doi: 10.34172/ajehe.5423
Review Article
The Ability of Vermicompost to Mitigate the Impacts of Salinity Stress on Soil Microbial Community: A Review
Halima Malal 1  , Mohamed Ait Hamza 2, Hicham Lakhtar 1, *
, Mohamed Ait Hamza 2, Hicham Lakhtar 1, * 
Author information:
1Microbial Biotechnology and Plant Protection Laboratory, Faculty of Science, Ibn Zohr University, Agadir, Morocco
2Laboratory of Biotechnology and Valorization of Natural Resources, University of Ibn Zohr, Faculty of Sciences, Agadir, Morocco
Abstract
Soil salinity is a major challenge in many developing countries, affecting soil fertility and crop productivity. Salinity directly affects the soil microbiome through osmotic pressure and ion toxicity, resulting in diminished microbial biomass and activity. Additionally, indirect repercussions involve reduced organic carbon inputs and aggregate stability, reducing microbial diversity and functions. Salinity induces a microbial community shift toward the abundance of halotolerant and halophile microorganisms. The use of organic amendments is a promising approach. Indeed, the application of vermicompost, with its rich nutrient and organic matter content, proves effective in counteracting the impact of salinity on the soil microbiome by providing available nutrients, decreasing the plasmolysis of cells by reducing the Na+/K+ and Na+/Ca2+ ratios, improving the soil texture, increasing the microbial diversity, and shifting the soil microbiome toward the abundance of beneficial soil microbiota. Despite these positive effects, carefully considering the initial EC of both soil and vermicompost and the applied quantity is crucial to ensuring maximum benefits. Overall, vermicompost holds considerable potential as a sustainable management strategy to mitigate the impact of salinity on soil microbiome, promoting overall soil health and enhancing crop production.
Keywords: Microbial community, Nutrients availability, Organic fertilizers, Soil health, Soil salinization, Sustainable agriculture,
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Malal H, Hamza MA, Lakhtar H. The ability of vermicompost to mitigate the impacts of salinity stress on soil microbial community: a review. Avicenna J Environ Health Eng. 2024; 11(1):55-62. doi:10.34172/ajehe.5423
1. Introduction
Soil salinization is a worldwide emerging problem, particularly in arid and semi-arid areas. It occurs when the soil contains an excessive amount of soluble salts, such as sodium (Na+), chloride (Cl-), potassium (K+), and sulfate (SO42-), with an electrical conductivity (EC) exceeding 4.0 dS/m (1). Soil salinization has adverse effects on plant growth, health, and crop production by reducing water uptake, altering nutrient availability, and disrupting soil aggregate stability (2). It occurs naturally through seawater intrusion or the release of salts from bedrock (3). However, human activities, such as using saline irrigation water and excessive application of fertilizers in semi-arid croplands, can also cause it. The phenomenon is exacerbated by climate change, leading to increased temperatures due to global warming and a lack of precipitation, causing a considerable rise in evapotranspiration rates and leading to salt accumulation and an increase in soil salinization (4). Almost 800 million hectares of land are affected by salinity, accounting for 6% of the total area of the planet. Of these lands, 63% are in Africa (5). Every year, salinity increases at a rate of 0.3 to 1.5 million hectares of farmland, leading to a reduction in crop production of over 20% (6). Experts predict that half of the world’s arable land will be affected by salinity by 2050 (1).
Assessing the response and adaptation of soil microbiomes to salinity stress has become increasingly important, as they are critical drivers of plant productivity, nutrient cycling, organic matter decomposition, and overall soil health. There are various approaches and technologies available to manage salinity stress. For instance, developing salt-tolerant cultivars and using chemical products such as gypsum, sulfuric acid, and calcium chloride can help leach salts from the soil (7). The use of plant growth-promoting microorganisms may also help reduce the negative impacts of salinity (8). However, these methods are often expensive, require advanced technology and good-quality water, and are not always affordable in developing countries. Therefore, low-cost and practical management tools should be developed to mitigate salinity stress. One promising approach is applying organic amendments (9). Their use can enhance soil aggregate stability and promote microbial growth and diversity, leading to improved aggregate formation and organic carbon stabilization, which can help plants resist salinity (10).
The relationship between salinity, soil microbiome, and organic amendments has stimulated growing interest in sustainable soil management practices such as vermicomposting, which involves the use of earthworms to convert various organic wastes into organic amendments. Studies have proven that vermicompost benefits plant growth, promotes soil health, and reduces plant diseases (11).
Several studies have shown that vermicompost can reduce the negative impacts of salinity on plants and soil (3,12-14). The unique qualities of vermicompost, including its increased nutrient levels and the enriching effect of earthworm gut passage on microbial communities (13), suggest heightened potential for addressing salinity-induced challenges. Despite the evident significance, the current literature reveals a notable gap in studies specifically investigating the influence of vermicompost on soil microbiome and its role in alleviating soil salinization. Therefore, a comprehensive review should be conducted to synthesize existing evidence, identify key research gaps, and pave the way for future investigations.
This review aims to consolidate relevant information on the capacity of vermicompost to alleviate salinity stress on the soil microbial community, knowing its mechanisms of action and efficiency. The first section of the review will be dedicated to understanding the damage caused by salinity to the soil microbial community. In the second section, we will identify the vermicompost characteristics and what makes it different from other organic amendments while indicating its impact on soil health. The third section will shed light on using vermicompost to overcome the effects of salinity on the microbial community of soil, trying to understand the mechanisms, the efficiency, and the limits of its application.
2. Data Collection
The papers for this review were collected by searching multiple databases, such as Scopus, Web of Science, and PubMed. Articles published in peer-reviewed journals were selected. The search strategy involved using the following keywords: soil salinization, vermicompost, and soil microbiome. A total of 100 articles met the inclusion criteria and were considered for analysis. The primary focus was on studies published from 2009 to 2023. Then, the papers were analyzed to choose the most relevant ones, reducing the number of papers considered for the review to 70. During our literature search on the effects of salinity on the soil microbiome, we observed a notable scarcity of research papers. This scarcity became even more pronounced when narrowing our focus on studies specifically addressing the impact of vermicompost on the soil microbiome.
3. The Effects of Salinity on Soil Microbiome
Soil salinity exerts osmotic stress and ion toxicity on the soil microbiome, reducing microbial biomass and activity and significantly shifting microbiome diversity and composition. This ultimately affects plant growth and crop yield by altering soil functions regulated by the soil microbiome.
Soil salinity can influence the soil microbiome negatively, reducing microbial biomass and activity, exerting a strong selective pressure on the microbial community, and leading to changes in microbial structure and diversity (15). The accumulation of solutes (Na+, Cl-, and HCO3-) enhances the risk of ion toxicity and osmotic pressure, causing the plasmolysis of cells, thus leading to the death of microorganisms and roots (15). Consequently, soil microbial activity and biomass decrease considerably in salinity conditions (16). The salinity stress can also negatively affect enzyme activity (17,18).
Salinity can affect the microbial community both directly and indirectly (Fig. 1). Direct effects are osmotic stress and ion toxicity, while indirect effects include changes in soil organic matter inputs, soil structure, nutrient availability, and aggregate stability. Salinity reduces plant growth, limiting carbon inputs, which decreases microbial activity and biomass (19). It can also induce the destruction of soil aggregation by causing the dispersion and detachment of soil particles from aggregates. Salinity-induced destruction of soil aggregation can harm microbial activity and diversity in soil as the soil aggregation provides a varied microhabitat for soil microbiota with different organic matter stabilization, oxygen fluxes, and water potential (20). Zhang et al (21) investigated the effect of salinity on soil health. The findings revealed an inverse correlation between the level of salinity and organic matter in the soil, mainly because of a decrease in organic matter inputs and an increase in the rate of decomposition and erosion (22). Another issue caused by soil salinity is the decrease in nutrient availability. In saline soils, the uptake and reduction of different forms of N are low, and the N2-fixation in modulating plants is also disturbed. As for the P, the high level of Na+ and the increase in soil pH lowers the P solubility. The same goes for the K+ uptake, which is inhibited in saline soil since the Na+ competes with K+ and interferes with its dependent process (23).
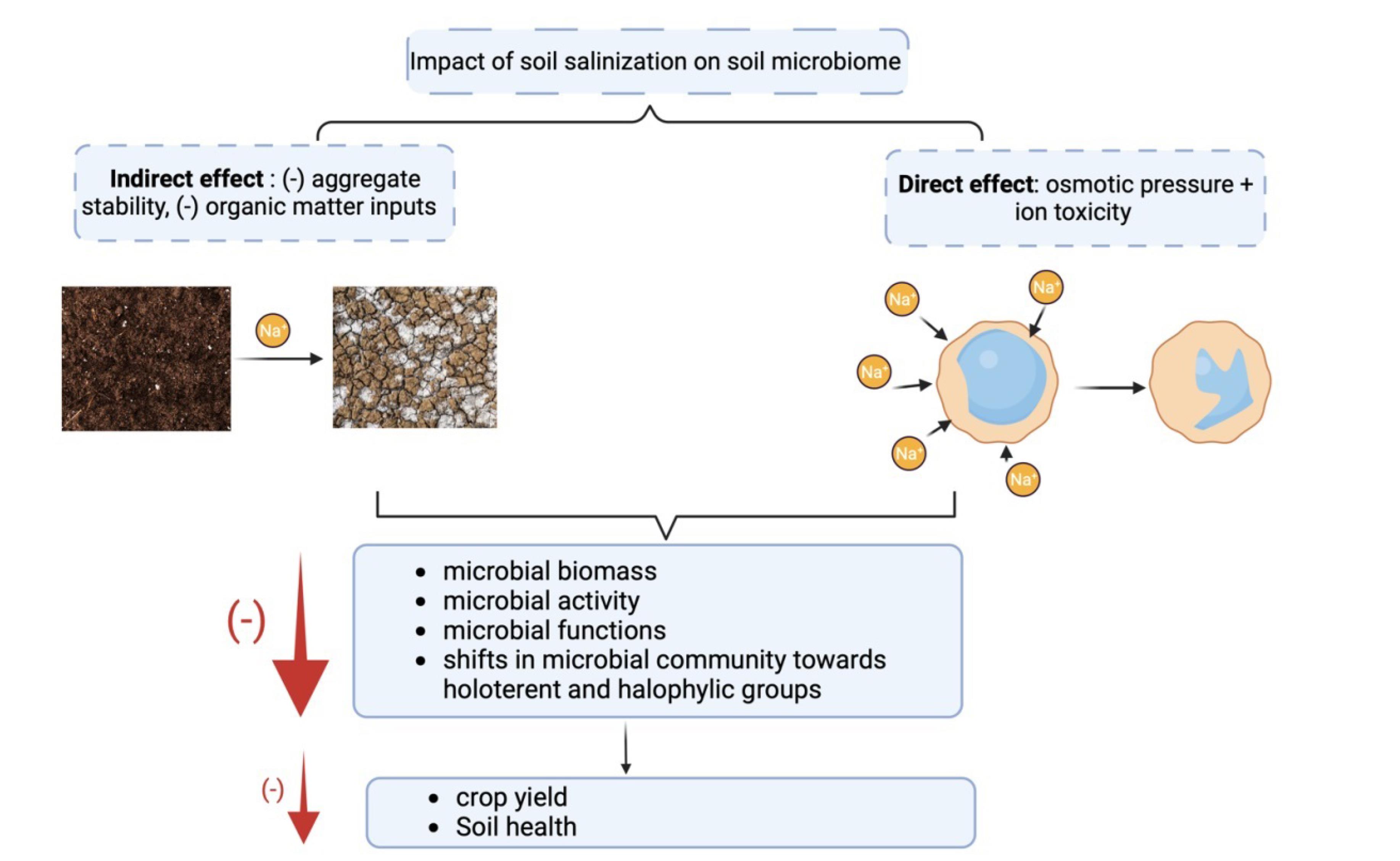
Fig. 1.
The Impact of Salinity on Soil Microbiome
.
The Impact of Salinity on Soil Microbiome
Salinity causes a shift in the microbial community by increasing the abundance of halophilic and halotolerant microbes and reducing the abundance of salt-sensitive microbes. At the phylum level, the relative abundance of Firmicutes, Bacteroidetes, and proteobacteria rises with elevated salinity, whereas the presence of Acidobacteria decreases. At the species level, Salinisphaerales, Xanthomonadales, andSyntrophobacteraleswere enriched in saline soils, while Nitrosomonadales, a salinity-sensitive species, decreased in saline conditions (19). The phyla Firmicutes was abundant in saline soils, probably because this phylum includes many halophilic and halotolerant species and other species capable of endospore synthesis. This property will allow them to survive in extreme environments and to increase their abundance after the death of other bacteria because of salinity exposure (24).
The mechanism of the impact of salinity on the fungal/bacterial ratio of the soil is complex. Studies have shown the dominance of fungi over bacteria in both saline (25) and non-saline soils (26) following exposure to salt stress. However, bacterial dominance over fungi has also been reported in soil affected by salinity (27). This indicates that the microbial response to salinity is complex and depends on various factors, including the soil legacy, which may favor bacterial dominance due to the presence of halophilic bacteria (25). Furthermore, it is worth noting that fungi have chitinous cell walls that enable them to resist osmotic pressure, which may explain their dominance in certain cases (24).
Salinity can change the abundance and structure of soil microbiome, consequently influencing their functions in soil. Zhang et al (21) evaluated the impact of salinity on soil health and microbial community composition. The study revealed that salinity affected bacterial communities by decreasing the abundance of Planctomycesand Archangium, two genera involved in the carbon cycle. The salinity also influenced the soil fungal community. For instance, the abundance of Hydropisphaera, efficient in lignin degradation,increased under salinity conditions. The effect of salinity also extended to the arbuscular mycorrhizal fungus Glomus which is involved in phosphorus and potassium uptakes of plants; in other words, its abundance decreased in saline conditions. The change that occurs in microbial diversity and community composition affects microbial function. A study conducted by Li et al (28) indicates that high salinity disturbs microbial diversity and impacts the abundance and structure of the nitrogen cycling bacterial communities, leading to the inhibition of nitrification-denitrification ammonification and nitrogen fixation processes.
Overall, salinity affects soil health and shifts microbial community, generating a poor soil structure, lower organic matter, and nutrient deficiencies. This will influence plant growth by restricting water infiltration and uptake, root penetration, and seedling emergence, resulting in yield loss (16).
4. The Use of Vermicompost to Improve Soil Characteristics
4.1. Vermicompost Characterization
Vermicompost, a product of the combined action of earthworms and microorganisms, is a stable product (29) characterized by high macro and micronutrient content (Table 1), high porosity and water holding capacity (30), higher microbial population, diversity, and enzymatic activity (30,31).
Table 1.
The Physico-chemical Characteristics of the Vermicompost
|
Source of vermicompost
|
Total C
(g/kg)
|
Total N
(g/kg)
|
P
(mg/kg)
|
K
(mg/kg)
|
Ca
(mg/kg)
|
Mg
(mg/kg)
|
Fe
(mg/kg)
|
Mn
(mg/kg)
|
pH
|
EC (dS/m)
|
Ref.
|
| Grape marc from Albariño variety |
154 ± 22.20 |
5.50 ± 0.97 |
751 ± 94 |
4364 ± 443 |
1236 ± 163 |
396 ± 72 |
44.40 ± 9.67 |
16.10 ±3.87 |
8.50 ± 0.13 |
0.35 ± 0.05 |
(24) |
| Grape marc from Mencía variety |
233 ± 14.50 |
8.93 ± 0.93 |
1418 ± 253 |
6466 ± 1708 |
2155 ± 244 |
673 ± 158 |
224 ± 61.30 |
49.20 ±14.40 |
7.55 ± 0.10 |
0.22 ± 0.05 |
(24) |
| Scotch broom |
471.44 ±0.45 |
36.42 ± 0.22 |
3070 ± 30 |
5990 ± 140 |
8990 ± 180 |
2900 ± 50 |
1890 ± 20 |
640 ± 10 |
6.60 ± 0.02 |
0.20 ± 00 |
(25) |
|
Ageratum conyzoides+ cow dung (1:1) |
- |
30.26 ± 1.40 |
- |
100350 +4510 |
87.67 ± 2.01 |
- |
- |
- |
7.32 ± 0.04 |
2.50 ± 0.02 |
(16) |
| Green waste |
- |
- |
- |
- |
- |
- |
- |
- |
7.70 ± 0.00 |
1.90 ± 0.00 |
(18) |
| Sewage sludge + cattle dung (2:3) |
- |
- |
- |
- |
- |
- |
- |
- |
5.90 ± 0.20 |
2.25 ± 0.05 |
(19) |
| Vegetable wastes |
- |
16.60 ± 0.50 |
- |
1800 ± 100 |
- |
- |
- |
- |
7.50 ± 0.02 |
- |
(20) |
| Food waste + cow dung |
- |
15.60 ± 0.30 |
- |
1500 ± 100 |
- |
- |
- |
- |
7.60 ± 0.01 |
- |
(20) |
| Crop residue + garden waste |
232 ± 0.00 |
13 ± 0.00 |
8900 ± 0.00 |
- |
- |
- |
- |
- |
6.20 ± 0.00 |
- |
(26) |
| Water hyacinth and paddy straw |
- |
14.10 |
9300 |
11200 |
- |
- |
- |
- |
- |
- |
(27) |
| Cow manure |
59.42 ± 0.00 |
7.68 ± 0.00 |
19090 ± 0.00 |
4130 ± 0.00 |
25100 ± 0.00 |
2920 ± 0.00 |
- |
- |
6.52 ± 0.00 |
2.19 ± 0.00 |
(21) |
A study by Zhou et al (32) revealed that vermicomposting enhanced the NPK content of pig manure vermicompost, resulting in a more stable product with a lower C/N ratio and higher TOC than compost. Additionally, vermicompost had a higher microbial population and enzymatic activity compared to compost. Similar findings were confirmed by another study conducted by Cai et al (33), which compared green waste composting and vermicomposting and revealed that vermicomposting produced a higher quality product with a higher level of N and P. Earthworms enhance the accessibility of organic matter to the soil microbiome by fragmenting organic matter and increasing surface area. As a result, decomposition occurs faster, leading to lower carbon-to-nitrogen ratios and stable products. Similarly, the bacterial diversity and richness were higher in the vermicompost (33). Furthermore, Huang et al (34) confirmed that the vermicompost of sewage sludge represented higher operational taxonomic units and bacterial diversity when compared to the compost.
Vermicompost is distinguished by a diverse microbial community. This community comprises phosphate solubilizers, plant growth-promoting bacteria, and free-living nitrogen-fixing organisms (34). During the vermicomposting of scotch broom, the microbial community showed increased taxonomic and phylogenetic bacterial diversity. Notably, a boost in functional diversity was observed through augmented nitrification, metabolic capacity, and the production of salicylic acid and streptomycin, known for their ability to promote plant resistance. Furthermore, this vermicompost contains the genus Devosia, known to aid in the fixation of nitrogen and release plant growth-promoting substances and antibiotics, and a variety of microorganisms that can produce plant cell-degrading enzymes, including members of the Chlorobi phylum, the Cellulomonodaceae family, and the Achromobacter genus (30). Huang et al (34) stated that the vermicompost of vegetable waste enhanced the bacterial and fungal diversity, while also promoting the growth of beneficial bacterial species such as Streptomyces spp. and fungal species like Paecilomyces spp.,Dactylaria biseptata, and Trichoderma spp.
5. Effects of Vermicompost on Soil Composition
Vermicompost application improves soil structure by enhancing the flocculation of clay minerals, leading to better soil aggregation. This results in reduced bulk density, increased soil aggregate stability, and improved porosity and aeration (35). Studies have demonstrated that vermicompost significantly enhances the total organic carbon content of the soil, making it a valuable source of stable organic matter. It improves physical, chemical, and biological properties by promoting soil organic matter. Applying vermicompost in crop rotations has been found to increase total organic carbon, particulate organic carbon, microbial biomass carbon, carbon mineralization rate, and recalcitrant carbon (36). Additionally, vermicompost enhances carbon storage and sequestration (37,38).
Moreover, vermicompost acts as a potent soil additive, enhancing nutrient status. Various studies confirm that adding vermicompost to soil increases nutrient content and availability, particularly NPK (39-44). Its longer nutrient retention time makes it an excellent source of nutrients for the soil (44). In lateritic soils, known for acidity, low organic matter, and high iron and aluminum oxides, vermicompost improves nutrient availability, enhances mineralizable N, and increases phosphorus solubility. This is crucial for overcoming nutrient disorders, especially the unavailability of phosphorus in lateritic soils (40,45).
Vermicompost positively influences microbial activity, biomass, and diversity. It increases the abundance of bacteria and Actinomycetes while decreasing fungi, restoring soil health by improving the bacterial/fungal ratio. Vermicompost also enhances enzymatic activity, including catalase and polyphenol oxidase activities (46). However, the impact on the microbial community can vary depending on soil characteristics, inheritance, and crop type (47–49).
The application of vermicompost extract induces a significant shift in microbial communities in the rhizosphere, either through direct colonization, biotic effects, or indirectly through the secretion of secondary metabolites and nutrients (49). According to Wu et al (50), vermicompost can enhance the diversity of bacterial and fungal communities in soil. It also shifts the microbial structure of soil by favoring the presence of Acidobacteria, Actinomycetes, Aspergillus, and Ascomycetes.
6. Use of Vermicompost in Moderating Salinity Effects on Soil Microbiome
Additionally, Liu et al (51) found that using vermicompost effectively mitigated the negative impacts of salinity on soil microbiome. This is achieved by enhancing the organic matter content and increasing aggregate stability, resulting in a notable shift in the bacterial community. The introduction of vermicompost resulted in a rise in the relative abundance of Skermanella and Sphingomonas. Additionally, using vermicompost amplified the presence of Arthrobacter, a plant growth-promoting rhizobacteria, and increased the abundance of Pedobacter. Both genera are recognized for their positive contributions to carbon, nitrogen, and potassium cycling. Their presence plays an important role in enhancing nutrient availability in saline soils, thereby aiding in mitigating salinity stress.
7. Possible Drawbacks of Vermicompost Application
There is no universally standardized approach for using vermicompost. The utilization of vermicompost typically depends on various factors, including the specific characteristics of the soil and the type of agricultural products being grown (52-54). Agricultural practices often consider factors such as soil type, nutrient content, pH, and EC levels (53). It is important to note that using vermicompost can lead to an increase in soil EC due to the presence of salts in the compost. This can be a concern for farmers, particularly in arid and semi-arid areas where soil salinity is already a threat to crop productivity (55).
The salt level of vermicompost depends on the nature of the feedstocks and the extent of their decomposition. Therefore, it is difficult to establish a universal rule for all types of vermicompost. For instance, vermicompost made using 70% sewage sludge and 30% cow dung had an EC of 4.16 dS/m(56), whereas vermicompost made from Halimeda gracilis seaweed and cow dung had an EC of 2.06 dS/m (57). Similarly, vermicompost made using 60% cow dung and 40% paper mill sludge had an EC of 5.77 dS/m (58).
The effect of vermicompost on soil salinity depends on the quantity applied and the EC of the soil. Demir (53) investigated the impact of vermicompost application on non-saline soil subjected to salinity stress. The study found that vermicompost increased soil EC values in low and medium Na salt levels (0 and 4 dS/m NaCl concentration), and this effect was correlated with the applied dose of vermicompost (0%, 2.5%, 5%). However, vermicompost reduced the EC values and the exchangeable sodium percentage under high salinity levels (8 dS/m NaCl concentration). On the other hand, the application of vermicompost to saline soils decreased the EC and ESP of the soil. A vermicompost made from crop residues (rice and maize) with an EC of 3.8 dS/m and an organic matter content of 42% was subjected to water stress in a study. The vermicompost was capable of reducing the EC of soil, exchangeable sodium percentage, and the sodium content, helping the soil recover from the combined effect of salinity and water deficit (59). Another study performed by Ding et al (60) indicated that the application of vermicompost with an EC of 3.2 dS/m to saline-sodic soil (EC = 7.3 dS/m, ESP = 24.5) reduced the EC and the ESP, thereby reducing soil salinity. Hence, assessing the EC of the soil before utilizing vermicompost is crucial. A soil with an initial EC below 4.0 dS/m may exhibit sensitivity to vermicompost application, while a saline soil with an EC exceeding 4.0 dS/m can accommodate the use of vermicompost without adverse effects on its overall EC.
It is also essential to consider the applied amount of organic amendment. Reddy and Crohn (55) tested the impact of compost salinity on plant growth. Briefly, a greenhouse experiment was conducted using 9 different types of compost applied to 3 crops (lettuce, tomato, and blueberry). Additionally, two rates of compost were applied to induce salinity levels similar to those associated with 10% and 25% yield reduction. Results indicate that applying compost at a high rate can effectively increase soil salinity and decrease plant growth like any other source of soil salinity; however, if applied at a moderate rate, compost was able to increase the plant growth of all treated crops.
The vermicompost application can improve the physico-chemical characteristics of the soil and help the soil mitigate the effect of salinity. These benefits can outweigh the cost of the added EC caused by vermicompost application, especially at low application rates (10-20 Mg/ha) (55). Vermicompost can also be applied at the same rate or even a lower rate (5 Mg/ha) (45).
8. Conclusion
In conclusion, salinity exerts a significant and diverse impact on the soil microbiome, causing direct reductions in microbial biomass and activity while inducing shifts in community composition. The complex interplay of osmotic stress, ion toxicity, and alterations in microbial structure and diversity has cascading effects on essential soil functions. Salinity negatively influences enzyme activity, disrupts soil aggregation, and alters the ratio of fungi to bacteria.
A growing number of recent studies have confirmed the effects of vermicompost, an eco-friendly and low-cost amendment, on the soil microbial community under salinity stress conditions. Vermicompost application enhanced microbial biomass and activity. Its effect can be explained either by direct colonization of vermicompost-origin taxa or by its nutrients and organic matter content. Although vermicompost application can improve salt-affected soils, it is wise to consider the possibility of increasing soil EC after vermicompost application, using the recommended rates and taking into consideration the types of crops and their sensitivity to salinity. Additionally, the initial value of EC of both soil and vermicompost can guarantee the optimal use of vermicompost.
Future research should delve deeper into this specific type of organic amendment, exploring its interactions with salinity and elucidating its nuanced effects on the soil microbial community. We recommend that subsequent studies should focus on various salty soils, utilizing vermicompost made from different feedstock materials and carefully assessing potential drawbacks to advance our understanding of sustainable soil management practices under salinity stress. Additionally, long-term studies are warranted to assess the sustained efficacy of vermicompost in different soil and climatic conditions.
Acknowledgements
The authors would like to thank sincerely Dr. Patricia Ann Lazicki and Dr. Cristina Lazcano for their valuable contributions and support during the writing of this manuscript.
Authors’ Contribution
Conceptualization: Halima Malal.
Data curation: Halima Malal.
Formal analysis: Halima Malal.
Investigation: Halima Malal.
Methodology: Hicham Lakhtar.
Project administration: Hicham Lakhtar.
Resources: Halima Malal.
Software: Mohamed Ait Hamza.
Supervision: Hicham Lakhtar.
Validation: Hicham Lakhtar.
Visualization: Mohamed Ait Hamza.
Writing–original draft: Halima Malal
Writing–review & editing: Hicham Lakhtar
Competing Interests
None.
Funding
No funding.
References
- Shrivastava P, Kumar R. Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 2015; 22(2):123-31. doi: 10.1016/j.sjbs.2014.12.001 [Crossref] [ Google Scholar]
- Stavi I, Thevs N, Priori S. Soil salinity and sodicity in drylands: a review of causes, effects, monitoring, and restoration measures. Front Environ Sci 2021; 9:712831. doi: 10.3389/fenvs.2021.712831 [Crossref] [ Google Scholar]
- Benazzouk S, Dobrev PI, Djazouli ZE, Motyka V, Lutts S. Positive impact of vermicompost leachate on salt stress resistance in tomato (Solanum lycopersicum L) at the seedling stage: a phytohormonal approach. Plant Soil 2020; 446(1):145-62. doi: 10.1007/s11104-019-04361-x [Crossref] [ Google Scholar]
- Ait-El-Mokhtar M, Baslam M, Ben-Laouane R, Anli M, Boutasknit A, Mitsui T. Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L) by the application of arbuscular mycorrhizal fungi and/or compost. Front Sustain Food Syst 2020; 4:131. doi: 10.3389/fsufs.2020.00131 [Crossref] [ Google Scholar]
- Kahime K, Ben Salem A, El Hidan A, Messouli M, Chakhchar A. Vulnerability and adaptation strategies to climate change on water resources and agriculture in Morocco: focus on Marrakech-Tensift-Al Haouz region. Int J Agric Environ Res 2018; 4(1):58-77. [ Google Scholar]
- Ben-Laouane R, Baslam M, Ait-El-Mokhtar M, Anli M, Boutasknit A, Ait-Rahou Y. Potential of native arbuscular mycorrhizal fungi, rhizobia, and/or green compost as alfalfa (Medicago sativa) enhancers under salinity. Microorganisms 2020; 8(11):1695. doi: 10.3390/microorganisms8111695 [Crossref] [ Google Scholar]
- El Hasini S, Halima OI, El Azzouzi M, Douaik A, Azim K, Zouahri A. Organic and inorganic remediation of soils affected by salinity in the Sebkha of Sed El Mesjoune – Marrakech (Morocco). Soil Tillage Res 2019; 193:153-60. doi: 10.1016/j.still.2019.06.003 [Crossref] [ Google Scholar]
- Bharti N, Barnawal D, Wasnik K, Tewari SK, Kalra A. Co-inoculation of Dietzianatronolimnaea and Glomus intraradices with vermicompost positively influences Ocimumbasilicum growth and resident microbial community structure in salt affected low fertility soils. Appl Soil Ecol 2016; 100:211-25. doi: 10.1016/j.apsoil.2016.01.003 [Crossref] [ Google Scholar]
- Jabeen N. Salinity stress alleviation by organic and inorganic fertilization. In: Hasanuzzaman M, Fujita M, Oku H, Nahar K, Hawrylak-Nowak B, eds. Plant Nutrients and Abiotic Stress Tolerance. Singapore: Springer; 2018. p. 437-76. 10.1007/978-981-10-9044-8_19.
- Szoboszlay M, Näther A, Liu B, Carrillo A, Castellanos T, Smalla K. Contrasting microbial community responses to salinization and straw amendment in a semiarid bare soil and its wheat rhizosphere. Sci Rep 2019; 9(1):9795. doi: 10.1038/s41598-019-46070-6 [Crossref] [ Google Scholar]
- Hussain N, Abbasi SA. Efficacy of the vermicomposts of different organic wastes as “clean” fertilizers: state-of-the-art. Sustainability 2018; 10(4):1205. doi: 10.3390/su10041205 [Crossref] [ Google Scholar]
- Akhzari D, Pessarakli M, Khedmati M. Effects of vermicompost and salinity stress on growth and physiological traits of Medicago rigidula L. J Plant Nutr 2016; 39(14):2106-14. doi: 10.1080/01904167.2016.1193609 [Crossref] [ Google Scholar]
- Ruiz-Lau N, Oliva-Llaven MA, Montes-Molina JA, Gutiérrez-Miceli FA. Mitigation of salinity stress by using the vermicompost and vermiwash. In: Bauddh K, Kumar S, Singh RP, Korstad J, eds. Ecological and Practical Applications for Sustainable Agriculture. Singapore: Springer; 2020. p. 345-56. 10.1007/978-981-15-3372-3_15.
- Xu L, Yan D, Ren X, Wei Y, Zhou J, Zhao H. Vermicompost improves the physiological and biochemical responses of blessed thistle (Silybum marianum Gaertn) and peppermint (Mentha haplocalyx Briq) to salinity stress. Ind Crops Prod 2016; 94:574-85. doi: 10.1016/j.indcrop.2016.09.023 [Crossref] [ Google Scholar]
- Abdul Rahman NS, Abdul Hamid NW, Nadarajah K. Effects of abiotic stress on soil microbiome. Int J Mol Sci 2021; 22(16):9036. doi: 10.3390/ijms22169036 [Crossref] [ Google Scholar]
- Tamilselvi SM, Thiyagarajan C, Elumalai V, Uthandi S. Microbial behavior, responses toward salinity stress, mechanism of microbe-mediated remediation for sustainable crop production. In: Santoyo G, Kumar A, Aamir M, Uthandi S, eds. Mitigation of Plant Abiotic Stress by Microorganisms. Academic Press; 2022. p. 103-27. 10.1016/b978-0-323-90568-8.00006-7.
- Leogrande R, Vitti C. Use of organic amendments to reclaim saline and sodic soils: a review. Arid Land Res Manag 2019; 33(1):1-21. doi: 10.1080/15324982.2018.1498038 [Crossref] [ Google Scholar]
- Yan N, Marschner P, Cao W, Zuo C, Qin W. Influence of salinity and water content on soil microorganisms. Int Soil Water Conserv Res 2015; 3(4):316-23. doi: 10.1016/j.iswcr.2015.11.003 [Crossref] [ Google Scholar]
- Dong Y, Zhang J, Chen R, Zhong L, Lin X, Feng Y. Microbial community composition and activity in saline soils of coastal agro-ecosystems. Microorganisms 2022; 10(4):835. doi: 10.3390/microorganisms10040835 [Crossref] [ Google Scholar]
- Gupta V. Microbes and soil structure. In: Gliński J, Horabik J, Lipiec J, eds. Encyclopedia of Agrophysics. Encyclopedia of Earth Sciences Series. Dordrecht: Springer; 2011. p. 470-2. 10.1007/978-90-481-3585-1_91.
- Zhang WW, Wang C, Xue R, Wang LJ. Effects of salinity on the soil microbial community and soil fertility. J Integr Agric 2019; 18(6):1360-8. doi: 10.1016/s2095-3119(18)62077-5 [Crossref] [ Google Scholar]
- Haj-Amor Z, Araya T, Kim DG, Bouri S, Lee J, Ghiloufi W. Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: a review. Sci Total Environ 2022; 843:156946. doi: 10.1016/j.scitotenv.2022.156946 [Crossref] [ Google Scholar]
- Bidalia A, Vikram K, Yamal G, Rao KS. Effect of salinity on soil nutrients and plant health. In: Akhtar MS, ed. Salt Stress, Microbes, and Plant Interactions: Causes and Solution: Volume 1. Singapore: Springer; 2019. p. 273-97. 10.1007/978-981-13-8801-9_13.
- Rath KM, Fierer N, Murphy DV, Rousk J. Linking bacterial community composition to soil salinity along environmental gradients. ISME J 2019; 13(3):836-46. doi: 10.1038/s41396-018-0313-8 [Crossref] [ Google Scholar]
- Kamble PN, Gaikwad VB, Kuchekar SR, Bååth E. Microbial growth, biomass, community structure and nutrient limitation in high pH and salinity soils from Pravaranagar (India). Eur J Soil Biol 2014; 65:87-95. doi: 10.1016/j.ejsobi.2014.10.005 [Crossref] [ Google Scholar]
- Rath KM, Maheshwari A, Bengtson P, Rousk J. Comparative toxicities of salts on microbial processes in soil. Appl Environ Microbiol 2016; 82(7):2012-20. doi: 10.1128/aem.04052-15 [Crossref] [ Google Scholar]
- Pankhurst CE, Yu S, Hawke BG, Harch BD. Capacity of fatty acid profiles and substrate utilization patterns to describe differences in soil microbial communities associated with increased salinity or alkalinity at three locations in South Australia. Biol Fertil Soils 2001; 33(3):204-17. doi: 10.1007/s003740000309 [Crossref] [ Google Scholar]
- Li X, Wang A, Wan W, Luo X, Zheng L, He G. High salinity inhibits soil bacterial community mediating nitrogen cycling. Appl Environ Microbiol 2021; 87(21):e01366-21. doi: 10.1128/aem.01366-21 [Crossref] [ Google Scholar]
- Zhou Y, Zhang D, Zhang Y, Ke J, Chen D, Cai M. Evaluation of temperature on the biological activities and fertility potential during vermicomposting of pig manure employing Eisenia fetida. J Clean Prod 2021; 302:126804. doi: 10.1016/j.jclepro.2021.126804 [Crossref] [ Google Scholar]
- Domínguez J, Aira M, Kolbe AR, Gómez-Brandón M, Pérez-Losada M. Changes in the composition and function of bacterial communities during vermicomposting may explain beneficial properties of vermicompost. Sci Rep 2019; 9(1):9657. doi: 10.1038/s41598-019-46018-w [Crossref] [ Google Scholar]
- Gusain R, Suthar S. Vermicomposting of invasive weed Ageratum conyzoids: assessment of nutrient mineralization, enzymatic activities, and microbial properties. Bioresour Technol 2020; 312:123537. doi: 10.1016/j.biortech.2020.123537 [Crossref] [ Google Scholar]
- Zhou Y, Li H, Guo W, Liu H, Cai M. The synergistic effect between biofertility properties and biological activities in vermicomposting: a comparable study of pig manure. J Environ Manage 2022; 324:116280. doi: 10.1016/j.jenvman.2022.116280 [Crossref] [ Google Scholar]
- Cai L, Gong X, Sun X, Li S, Yu X. Comparison of chemical and microbiological changes during the aerobic composting and vermicomposting of green waste. PLoS One 2018; 13(11):e0207494. doi: 10.1371/journal.pone.0207494 [Crossref] [ Google Scholar]
- Huang K, Li F, Wei Y, Chen X, Fu X. Changes of bacterial and fungal community compositions during vermicomposting of vegetable wastes by Eisenia foetida. Bioresour Technol 2013; 150:235-41. doi: 10.1016/j.biortech.2013.10.006 [Crossref] [ Google Scholar]
- Kumari R, Bhatnagar S, Deepali Deepali, Mehla N, Vashistha A. Potential of organic amendments (AM fungi, PGPR, vermicompost and seaweeds) in combating salt stress a review. Plant Stress 2022; 6:100111. doi: 10.1016/j.stress.2022.100111 [Crossref] [ Google Scholar]
- Sarma B, Farooq M, Gogoi N, Borkotoki B, Kataki R, Garg A. Soil organic carbon dynamics in wheat - green gram crop rotation amended with vermicompost and biochar in combination with inorganic fertilizers: a comparative study. J Clean Prod 2018; 201:471-80. doi: 10.1016/j.jclepro.2018.08.004 [Crossref] [ Google Scholar]
- Banashree S, Smrita B, Nath DJ, Nirmali G. Temporal responses of soil biological characteristics to organic inputs and mineral fertilizers under wheat cultivation in inceptisol. Arch Acker Pflanzenbau Bodenkd 2017; 63(1):35-47. doi: 10.1080/03650340.2016.1179385 [Crossref] [ Google Scholar]
- Ngo PT, Rumpel C, Doan Thu T, Henry-des-Tureaux T, Dang DK, Jouquet P. Use of organic substrates for increasing soil organic matter quality and carbon sequestration of tropical degraded soil: a 3-year mesocosms experiment. Carbon Manag 2014; 5(2):155-68. doi: 10.1080/17583004.2014.912868 [Crossref] [ Google Scholar]
- Das D, Abbhishek K, Banik P, Swain DK. Comparative evaluation of changes in soil bio-chemical properties after application of traditional and enriched vermicompost. Environ Technol Innov 2022; 28:102956. doi: 10.1016/j.eti.2022.102956 [Crossref] [ Google Scholar]
- Pramanik P, Ghosh GK, Chung YR. Changes in nutrient content, enzymatic activities and microbial properties of lateritic soil due to application of different vermicomposts: a comparative study of ergosterol and chitin to determine fungal biomass in soil. Soil Use Manag 2010; 26(4):508-15. doi: 10.1111/j.1475-2743.2010.00304.x [Crossref] [ Google Scholar]
- Nurhidayati N, Machfudz M, Murwani I. Direct and residual effect of various vermicompost on soil nutrient and nutrient uptake dynamics and productivity of four mustard Pak-Coi (Brassica rapa L) sequences in organic farming system. Int J Recycl Org Waste Agric 2018; 7(2):173-81. doi: 10.1007/s40093-018-0203-0 [Crossref] [ Google Scholar]
- Sharma RP, Datt N, Chander G. Effect of vermicompost, farmyard manure and chemical fertilizers on yield, nutrient uptake and soil fertility in okra (Abelmoschus esculentus)-onion (Allium cepa) sequence in wet temperate zone of Himachal Pradesh. J Indian Soc Soil Sci 2009; 57(3):357-61. [ Google Scholar]
- Singh M, Wasnik K. Effect of vermicompost and chemical fertilizer on growth, herb, oil yield, nutrient uptake, soil fertility, and oil quality of rosemary. Commun Soil Sci Plant Anal 2013; 44(18):2691-700. doi: 10.1080/00103624.2013.813532 [Crossref] [ Google Scholar]
- Tammam AA, Rabei Abdel Moez Shehata M, Pessarakli M, El-Aggan WH. Vermicompost and its role in alleviation of salt stress in plants–I Impact of vermicompost on growth and nutrient uptake of salt-stressed plants. J Plant Nutr 2023; 46(7):1446-57. doi: 10.1080/01904167.2022.2072741 [Crossref] [ Google Scholar]
- Oo AN, Iwai CB, Saenjan P. Soil properties and maize growth in saline and nonsaline soils using cassava-industrial waste compost and vermicompost with or without earthworms. Land Degrad Dev 2015; 26(3):300-10. doi: 10.1002/ldr.2208 [Crossref] [ Google Scholar]
- Zhao F, Zhang Y, Li Z, Shi J, Zhang G, Zhang H. Vermicompost improves microbial functions of soil with continuous tomato cropping in a greenhouse. J Soils Sediments 2020; 20(1):380-91. doi: 10.1007/s11368-019-02362-y [Crossref] [ Google Scholar]
- Allard SM, Walsh CS, Wallis AE, Ottesen AR, Brown EW, Micallef SA. Solanum lycopersicum (tomato) hosts robust phyllosphere and rhizosphere bacterial communities when grown in soil amended with various organic and synthetic fertilizers. Sci Total Environ 2016; 573:555-63. doi: 10.1016/j.scitotenv.2016.08.157 [Crossref] [ Google Scholar]
- Gao Y, Tian Y, Liang X, Gao L. Effects of single-root-grafting, double-root-grafting and compost application on microbial properties of rhizosphere soils in Chinese protected cucumber (Cucumis sativus L) production systems. Sci Hortic 2015; 186:190-200. doi: 10.1016/j.scienta.2015.02.026 [Crossref] [ Google Scholar]
- Munoz-Ucros J, Panke-Buisse K, Robe J. Bacterial community composition of vermicompost-treated tomato rhizospheres. PLoS One 2020; 15(4):e0230577. doi: 10.1371/journal.pone.0230577 [Crossref] [ Google Scholar]
- Wu Q, Zhang J, Liu X, Chang T, Wang Q, Shaghaleh H. Effects of biochar and vermicompost on microorganisms and enzymatic activities in greenhouse soil. Front Environ Sci 2023; 10:1060277. doi: 10.3389/fenvs.2022.1060277 [Crossref] [ Google Scholar]
- Liu M, Wang C, Wang F, Xie Y. Vermicompost and humic fertilizer improve coastal saline soil by regulating soil aggregates and the bacterial community. Arch Acker Pflanzenbau Bodenkd 2019; 65(3):281-93. doi: 10.1080/03650340.2018.1498083 [Crossref] [ Google Scholar]
- Bidabadi SS, Dehghanipoodeh S, Wright GC. Vermicompost leachate reduces some negative effects of salt stress in pomegranate. Int J Recycl Org Waste Agric 2017; 6(3):255-63. doi: 10.1007/s40093-017-0173-7 [Crossref] [ Google Scholar]
- Demir Z. Alleviation of adverse effects of sodium on soil physicochemical properties by application of vermicompost. Compost Sci Util 2020; 28(2):100-16. doi: 10.1080/1065657x.2020.1789011 [Crossref] [ Google Scholar]
- Wang L, Sun X, Li S, Zhang T, Zhang W, Zhai P. Application of organic amendments to a coastal saline soil in north China: effects on soil physical and chemical properties and tree growth. PLoS One 2014; 9(2):e89185. doi: 10.1371/journal.pone.0089185 [Crossref] [ Google Scholar]
- Reddy N, Crohn DM. Compost induced soil salinity: a new prediction method and its effect on plant growth. Compost Sci Util 2012; 20(3):133-40. doi: 10.1080/1065657x.2012.10737038 [Crossref] [ Google Scholar]
- Ludibeth SM, Marina IE, Vicenta EM. Vermicomposting of sewage sludge: earthworm population and agronomic advantages. Compost Sci Util 2012; 20(1):11-7. doi: 10.1080/1065657x.2012.10737016 [Crossref] [ Google Scholar]
- Ananthavalli R, Ramadas V, John Paul JA, Karunai Selvi B, Karmegam N. Seaweeds as bioresources for vermicompost production using the earthworm, Perionyx excavatus (Perrier). Bioresour Technol 2019; 275:394-401. doi: 10.1016/j.biortech.2018.12.091 [Crossref] [ Google Scholar]
- Kumar Badhwar V, Singh S, Singh B. Biotransformation of paper mill sludge and tea waste with cow dung using vermicomposting. Bioresour Technol 2020; 318:124097. doi: 10.1016/j.biortech.2020.124097 [Crossref] [ Google Scholar]
- Hafez EM, Omara AE, Alhumaydhi FA, El-Esawi MA. Minimizing hazard impacts of soil salinity and water stress on wheat plants by soil application of vermicompost and biochar. Physiol Plant 2021; 172(2):587-602. doi: 10.1111/ppl.13261 [Crossref] [ Google Scholar]
- Ding Z, Kheir AM, Ali OA, Hafez EM, ElShamey EA, Zhou Z. A vermicompost and deep tillage system to improve saline-sodic soil quality and wheat productivity. J Environ Manage 2021; 277:111388. doi: 10.1016/j.jenvman.2020.111388 [Crossref] [ Google Scholar]