Avicenna J Environ Health Eng. 8(2):110-115.
doi: 10.34172/ajehe.2021.14
Original Article
Evaluation of Natural and Chemical Coagulants Performance in Treatment of Municipal Wastewater of Behshahr City
Sakineh Tabaki 1, Fatemeh Ardestani 1, * 
Author information:
1Department of Chemical Engineering, Qaemshahr Branch, Islamic Azad University, Qaemshahr, Iran
Abstract
Municipal wastewater is one of the largest volumes of wastewater which contains various organic compounds from proteins and fats to carbohydrates and nucleic acids. Municipal wastewater of Behshahr city (Mazandaran, Iran) was evaluated using aluminum sulfate and iron chloride as chemical coagulants and pectin and sodium alginate as natural ones. Biological oxygen demand was investigated at different temperatures and coagulant concentrations. The fraction of full factorial statistical method and Qualitek-4 software were applied for designing experiments and analyzing the results to determine the optimal conditions for achieving the highest reduction in wastewater organic load and biological oxygen demand. In the case of biological oxygen demand, the optimal condition was achieved at 25°C, using 1, 0.02, 1.5 and 1 g/L of aluminum sulfate, sodium alginate, iron chloride and pectin, respectively. Under the optimal condition, the percentage of biological oxygen demand reduction was equal to 34.5%. Maximum BOD removal of 40.9% was obtained at 25°C using aluminum sulfate, sodium alginate, iron chloride, and pectin at concentrations of 0.6, 0.02, 1.5, and 6 g/L, respectively. The contribution of aluminum sulfate, sodium alginate, iron chloride, and pectin concentration in biological oxygen demand removal of the studied wastewater was 3.8%, 22.4%, 16.3%, and 14.4%, respectively. Coagulation temperature and aluminum sulfate concentration with approximately 42.7% and 3.8% contribution values were determined as the most and the least effective factors in biological oxygen demand reduction.
Keywords: Biological oxygen demand, Coagulation, Municipal wastewater, Taguchi approach
Copyright and License Information
© 2021 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
Homes are the main sources of municipal wastewater production. Municipal wastewater comes from health centers, restaurants, offices, collected surface water, rainwater, and all types of household wastewater. Wastewater containing effluents from washing machines, toilets, baths, and dishwashers is also classified as municipal wastewater (1).
Municipal wastewater is one of the best types of wastewater in terms of the type of solutes and suspended particles that can be treated with simple methods and returned to the consumption cycle. Therefore, disposing of municipal wastewater without having and considering a wastewater treatment system is a waste of resources. A high volume of wastewater is generated which includes many different types of organic compounds from proteins and fats to carbohydrates and nucleic acids. If this wastewater enters the environment without going through the treatment process and reducing the organic load, it will cause environmental pollution. The biochemical oxygen demand (BOD) level of municipal wastewater is different and the amount of its organic matter can be the largest and most important factor affecting the environment and natural ecosystem. Therefore, the separation of organic compounds in municipal wastewater will not only help reduce the BOD of wastewater but will also be of great help in preserving the natural ecosystem, plants, and aquatic organisms (2).
Some organic pollutants are resistant to decomposition and their presence is toxic and dangerous to aquatic life. As a result, eliminating or reducing their concentration is necessary to maintain the health of the environment and organisms, which must be done using appropriate methods. The presence of organic matter in water reduces the amount of its dissolved oxygen content. Coagulation was introduced as an effective method to remove organic pollutants from different types of wastewaters (3).
Some natural and chemical coagulants have been studied in wastewater treatment process. Chitosan and activated carbon nano-composite showed good removal efficiency for ammonia, phosphate, and nitrite from aquaculture wastewater (4). The results of a study by Isik et al indicated that the electro-oxidation method using activated carbon cloth electrodes had good efficiency in the removal of pollutants from textile dye bath wastewater (5). Biofilters as natural method showed acceptable removal performance in different wastewaters (6). Polyferric chloride was effective in the removal of total organic carbon, NH4+-N, and PO43−-P from municipal wastewater (7). Using a mixture of some coagulating agents such as aluminum sulfate, ferrous sulfate, and ferric sulfate in municipal treatment plant has led to BOD removal efficiency of 55% (8). Recently, much attention has been paid to the treatment of effluents containing high amounts of organic matter using natural coagulants (9). Aloe steudneri gel as a natural coagulant showed a high efficiency of 91.57% in BOD removal from textile wastewater (10). Pectin is a heteropolysaccharide material known as a key agent for plant development including immunity, growth, and morphological changes and some stabilizing applications in the food industry. Pectin has been introduced as a coagulant which could be used in water and wastewater treatment (11). Sodium alginate is the sodium salt of alginic acid, a natural polysaccharide found in brown algae, which is used as a thickener and stabilizer in the food production industry. Biodegradable stable gels of sodium alginate are made by cross-linking in the presence of divalent cations such as Ca2+. These gels are introduced as sustainable materials for cell immobilization and encapsulation.
The present study evaluated biological oxygen demand reduction in municipal wastewater of Behshahr city (Mazandaran, Iran) using aluminum sulfate, sodium alginate, iron chloride, and pectin using the fraction of full factorial statistical method and Qualitek-4 software.
2. Materials and Methods
2.1. Materials
Aluminum sulfate hydrate (Al2(SO4)3. H2O) 98% with a molecular weight of 342.15, sodium alginate (C6H9NaO7), iron (III) chloride (FeCl3) 97% anhydrous powder with a molecular weight of 162.20, and other chemicals were prepared from Merck Millipore Co. (Germany). Pectin (poly-D-galacturonic acid methyl ester) was purchased from Sigma Aldrich Co. (USA).
2.2. Sampling and Preparation of Samples
The municipal wastewater used in this research was prepared from the entrance of the wastewater treatment plant of Behshahr city. Behshahr is one of the cities of Mazandaran province and one of the oldest cities in Iran, with a population of 97 402 people according to the latest census in 2016. Behshahr city has a temperate and humid climate as it is surrounded by mountains and sea. Heavy rain and high humidity are observed in the southern parts of the city due to its mountainous terrain, high altitude, colder and mountainous climate, and rainy and snowy winters.
A total of 15 samples of municipal wastewater, each with a volume of 1 L, were taken from the inlet tank of wastewater treatment plant of Behshahr in spring for 15 consecutive days and placed in glass bottles. Then, the samples were thoroughly mixed with each other and finally a mixed sample with a volume of 5 L was prepared as the final wastewater sample. The samples were used immediately and without delay for the flocculation process.
2.3. Experiment Design
Experiments were designed to simultaneously evaluate the performance of the desired factors using the fraction of full factorial statistical method and Qualitek-4 software. Analysis of experimental results using Taguchi method and signal-to-noise analysis finally led to the determination of the optimal conditions in terms of the mentioned factors. Flocculation temperature and concentration of flocculants including aluminum sulfate, sodium alginate, iron chloride, and pectin in 4 different levels (Table 1) were considered as influential variables to determine an L-16 orthogonal array for designing experiments using Qualitek-4 software (Table 2).
Table 1.
Key Factors and Their Levels in Optimization of BOD Reduction in Municipal Wastewater of Behshahr City
|
Factor
|
Level 1
|
Level 2
|
Level 3
|
Level 4
|
| Temperature (°C) |
20 |
25 |
30 |
35 |
| Aluminum sulfate concentration (g/L) |
0.6 |
1 |
1.5 |
2 |
| Sodium alginate concentration (g/L) |
0.01 |
0.02 |
0.03 |
0.04 |
| Iron chloride concentration (g/L) |
0.4 |
0.8 |
1.5 |
3 |
| Pectin concentration (g/L) |
1 |
2 |
4 |
6 |
Table 2.
The Layout of the L-16 Orthogonal Arrays in Optimization of BOD Reduction in Municipal Wastewater of Behshahr City
|
Factor
|
Temperature (°C)
|
Aluminum Sulfate Concentration (g/L)
|
Sodium Alginate Concentration (g/L)
|
Iron Chloride Concentration (g/L)
|
Pectin
Concentration (g/L)
|
|
Trial
|
|
|
|
|
|
| 1 |
1 |
1 |
1 |
1 |
1 |
| 2 |
1 |
2 |
2 |
2 |
2 |
| 3 |
1 |
3 |
3 |
3 |
3 |
| 4 |
1 |
4 |
4 |
4 |
4 |
| 5 |
2 |
1 |
2 |
3 |
3 |
| 6 |
2 |
2 |
1 |
4 |
4 |
| 7 |
2 |
3 |
4 |
1 |
1 |
| 8 |
2 |
4 |
3 |
2 |
2 |
| 9 |
3 |
1 |
3 |
4 |
4 |
| 10 |
3 |
2 |
4 |
3 |
3 |
| 11 |
3 |
3 |
1 |
2 |
2 |
| 12 |
3 |
4 |
2 |
1 |
1 |
| 13 |
4 |
1 |
4 |
2 |
2 |
| 14 |
4 |
2 |
3 |
1 |
1 |
| 15 |
4 |
3 |
2 |
4 |
4 |
| 16 |
4 |
4 |
1 |
3 |
3 |
2.4. Flocculation Process
Samples were prepared by adding 100 mL of wastewater solution into each of 16 separate bottles. While stirring the samples at 50 rpm, aluminum sulfate, sodium alginate, iron chloride, and pectin were added to each wastewater sample according to the designed template (Tables 1 and 2). The bottles were then stored in appropriate incubators at the specified temperature (Tables 1 and 2) for 5 hours. The contents of 16 bottles were passed through filter paper separately and the filtered solutions were used for BOD analysis.
2.5. BOD Analysis
In order to prepare a control sample, some distilled water was poured into a 300 mL bottle. Then, 2 mL of manganese sulfate solution and 2 mL of alkaline iodine solution were evacuated by pipette under the water surface and was shaken several times until manganese hydroxide precipitate appeared and the precipitate settled. Then, 2 mL of concentrated sulfuric acid was added to the bottle. After stirring and dissolving all the sediments, 1 to 2 mL of starch solution was added to the sample and titrated with 0.030 N sodium thiosulfate solution until a blue color appeared.
Experimental treatments were performed by pouring 5 mL of the flocculated wastewater samples into BOD measuring tubes and filling the tubes with dilution water. The samples were incubated for 20 days at 20°C. The oxygen content of the control bottle was measured after 15 minutes and for other treatments, it was measured after 5 days.
For BOD measurement, 1 mL of the primary wastewater sample (as a control sample) and 1 mL of each 16 purified samples (wastewater samples with coagulants according to Tables 1 and 2) were added to the BOD measuring tube and filled with oxygen-saturated distilled water. Then, 1 mL of phosphate buffer (to maintain pH), magnesium sulfate, calcium chloride, and iron chloride solutions per 1 L of distilled water were added to each sample.
Winkler method was used for BOD assessment. The starch adhesive was prepared by dissolving 5 g of starch powder in 800 mL of boiling water and increasing its volume to 1 L. In Winkler method, first, 2 mL of manganese sulfate solution was added to the samples to stabilize the dissolved oxygen, and then, 2 mL of alkaline iodide solution and starch adhesive were added. With the addition of reagents, a brownish-white precipitate was obtained. After 3 minutes, the solution in the bottles was acidified by adding 2 mL of concentrated sulfuric acid. As the sediment dissolved, the transparency of the sample was lost and the released iodine was slowly dispersed in the sample. Finally, titration was performed using sodium thiosulfate solution to achieve a matt yellow color. Titration with sodium thiosulfate was continued until the blue color faded. After 5 days of incubation, BOD was measured for each sample according to Winkler method. Using the difference in the amount of dissolved oxygen in the two prototypes and the incubated sample, BOD of each sample was determined (1,11).
3. Results and Discussion
Based on the results, the amount of BOD in the wastewater sample of Behshahr city was equal to 295 mg/L. In this study, the main purpose was to reduce BOD in the wastewater of Behshahr city by adding coagulants associated with different coagulation temperatures.
3.1. BOD Removal Results
The percentage of BOD reduction and S/N ratio calculated by the software are presented in Table 3. The output variable used in statistical analysis is the percentage of BOD reduction, which is a measurable physical quantity. The larger this quantity, the more desirable the wastewater treatment rate. The analysis considered in this research is signal-to-noise analysis using S/N ratio.
Table 3.
Experimental Results and Calculated S/N in Optimization of BOD Reduction in Municipal Wastewater of Behshahr City (Mazandaran, Iran)
|
Factor
|
BOD Reduction (%)
|
|
Trial
|
Repeat 1
|
Repeat 2
|
S/N
|
| 1 |
9.085 |
9.078 |
19.084 |
| 2 |
10.193 |
10.186 |
20 |
| 3 |
7.946 |
7.952 |
16.901 |
| 4 |
9.495 |
9.498 |
19.084 |
| 5 |
40.9 |
40.902 |
32.041 |
| 6 |
21.56 |
21.57 |
26.444 |
| 7 |
18.858 |
18.861 |
25.105 |
| 8 |
23.84 |
23.83 |
27.234 |
| 9 |
7.925 |
7.932 |
16.901 |
| 10 |
23.18 |
23.18 |
27.234 |
| 11 |
19.97 |
19.96 |
25.575 |
| 12 |
17.94 |
17.95 |
24.608 |
| 13 |
14.54 |
14.53 |
22.922 |
| 14 |
12.68 |
12.685 |
21.583 |
| 15 |
20.905 |
20.901 |
26.02 |
| 16 |
16.105 |
16.108 |
24.082 |
3.2. Average Effect of Each Factor on BOD Removal
The mean S/N ratio of different levels of each studied factors including temperature, aluminum sulfate, sodium alginate, pectin, and iron chloride concentrations are presented in Fig. 1. The average values of S/N for experiments performed using levels 1 to 4 of the temperature factor are equal to 18.8, 27.7, 23.6, and 23.7, respectively. Therefore, the highest BOD removal was obtained at level 2 of the temperature factor that is 25°C. Increasing the temperature from level 1 to level 2 increased BOD removal value by 47%, while by increasing temperature level 3, BOD removal has experienced a 15% decrease. By increasing the temperature from level 3 to level 4, no significant change was observed in BOD removal value. It seems that at temperatures above 30°C, this factor does not have stronger effects on flocculation process; however, temperature plays an important and key role in the efficiency of the coagulation and flocculation process.
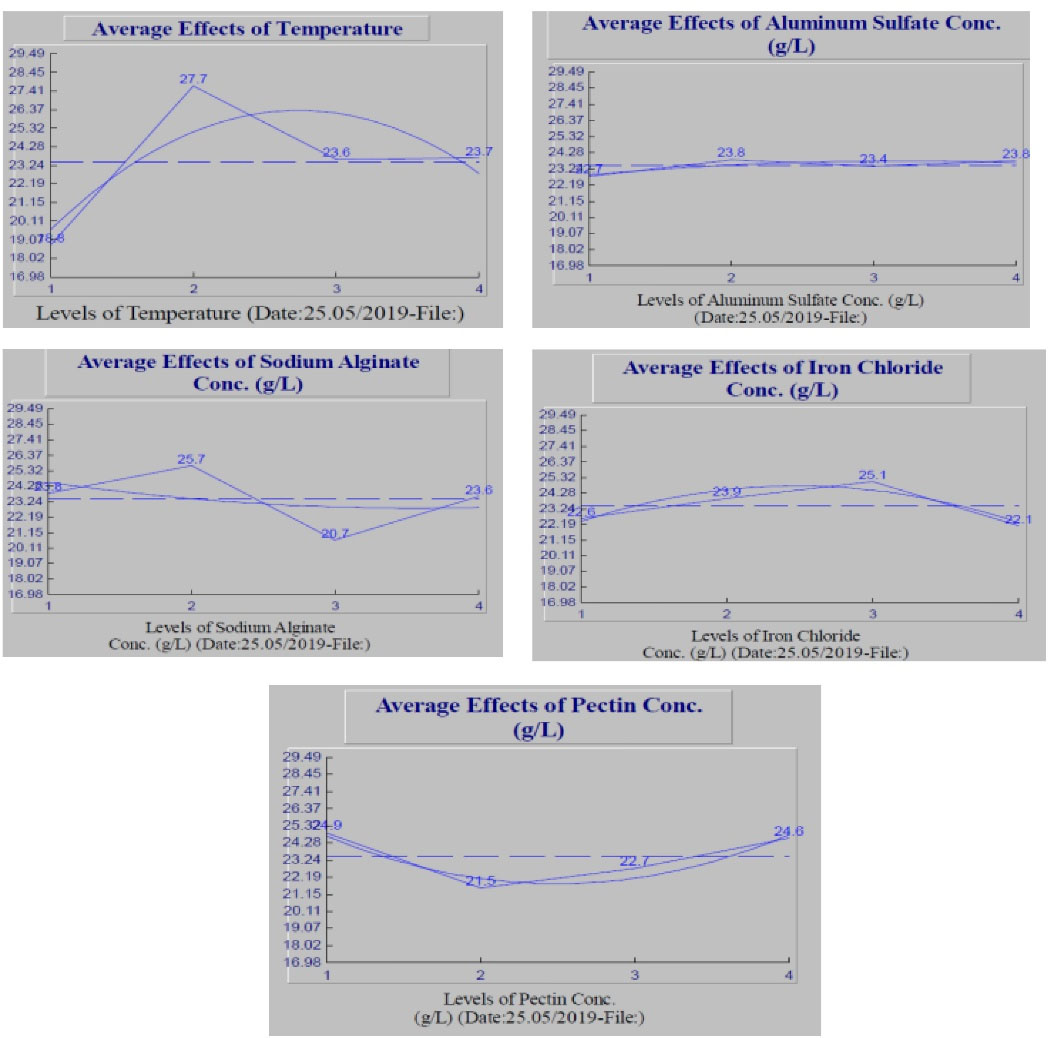
Figure 1.
The Mean S/N Ratio of Temperature, Aluminum Sulfate, Sodium Alginate, Iron Chloride and Pectin Concentrations in Optimization of BOD Reduction in Municipal Wastewater Treatment Plant of Behshahr
.
The Mean S/N Ratio of Temperature, Aluminum Sulfate, Sodium Alginate, Iron Chloride and Pectin Concentrations in Optimization of BOD Reduction in Municipal Wastewater Treatment Plant of Behshahr
Basically, by increasing the temperature, this efficiency increases. As the temperature decreased to close to zero, serious coagulation problems occurred and a decrease in the aggregation rate was observed (12). For this reason, the amount of coagulants used in treatment plants is higher in winter than in summer. According to the results of this study, the optimal temperature in order to achieve the highest aggregation rate and BOD removal efficiency was 25°C. A further increase in temperature may be associated with a decrease in intermolecular forces and a decrease in particle coagulation. However, in the analysis performed to determine the final optimal conditions, interactions of all five factors are considered. However, in these diagrams, the effect of each factor is expressed separately and without considering the interactions of other factors on it (13).
Fig. 1 shows that in the case of aluminum sulfate concentration, the highest BOD removal value was obtained at levels 2 and 4 of this factor. The average values of S/N for levels 1 to 4 of this factor are equal to 22.7, 23.8, 23.4, and 23.8, respectively. The difference between the lowest and the highest BOD removal value of this factor is just about 4.7%; therefore, it seems that the concentration of aluminum sulfate has very little effect on the aggregation rate. However, level 2 of this factor (1 g/L of aluminum sulfate) indicates optimal conditions. Adding a coagulant such as aluminum sulfate to the effluent does not charge the colloidal particles, and as these particles come closer together, larger particles are formed. Aluminum sulfate is hydrolyzed in wastewater to form ions or hydroxides or charged hydroxides. These large charged molecules adhere to the colloidal particles, reducing the potential difference between the diffusion phase and its surroundings (zeta potential). In fact, the zeta potential is the main repulsive factor between colloidal particles, and reducing this potential with the help of coagulants provides the necessary possibility to create van der Waals force and causes the particles to stick to each other. Therefore, the main cause of charge removal of colloidal particles is not metal ions, but the products of their hydrolysis. The coagulation process is eventually completed by flocculation, and larger particles begin to settle. Aluminum sulfate in the wastewater sample is converted to aluminum hydroxide and eventually larger flocs or aggregates are formed (14).
Sodium alginate is a linear polysaccharide and a copolymer made from brown seaweed. Sodium alginate is a cheap and available biopolymer that occurs naturally in brown algae and has received much attention due to its biodegradability, hydrophilic properties, gel formation, and pH sensitivity. Sodium alginate is soluble in water and is obtained by dissolving it in a liquid with a high viscosity. As shown in Fig. 1, the best result for this factor is seen at level 2 (0.02 g/L). In fact, selecting an appropriate concentration of sodium alginate in the flocculation process increases the efficiency by about 24%, while this amount for aluminum sulfate was about 4.5%. Pectin is a heteropolysaccharide found in the cell wall of a plant. Pectins are very diverse in terms of formation, chain length, and also in the arrangement of the monosaccharides that make them up. It is obtained by the decomposition of propectin and turns into a gel when exposed to an acidic environment. The best performance of pectin in the flocculation process is at level 1, i.e., the concentration of 1 g/L. According to Fig. 1, the maximum average S/N ratio for iron chloride concentration factor was obtained at its level 3, (concentration of 1.5 g/L). The functional mechanism of iron chloride in the coagulation process is similar to that of aluminum sulfate. Iron chloride reacts with calcium bicarbonate or calcium hydroxide after being added to the effluent sample to form iron hydroxide, which is known to cause aggregation of colloidal particles (15).
3.3. Optimal Condition of BOD Removal
Optimal condition to achieve the highest efficiency of BOD removal was determined using the software analysis tools. The best temperature was 25°C and the best concentrations of aluminum sulfate, sodium alginate, iron chloride, and pectin were evaluated to be 1, 0.02, 1.5, and 1 g/L, respectively. As it turns out, the optimal state is not obtained in any of the 16 designed experiments. Under the optimal condition, the percentage of BOD reduction was equal to 34.5%. Maximum BOD removal of 40.9% was obtained at 25°C using aluminum sulfate, sodium alginate, iron chloride, and pectin concentration of 0.6, 0.02, 1.5, and 6 g/L, respectively. The results of the present are more satisfactory than the findings of the study by Czerwionka et al that reported an average removal efficiency of 34% for organic matter using some organic and inorganic coagulants (16). Moreover, results show that the most effective factor in BOD reduction is coagulating temperature as emphasized in some other works (17). However, in a study by Berg, a 68% reduction in BOD has been reported using ferric chloride in an anaerobic fluidized bed reactor, indicating a higher efficiency in BOD reduction compared to the results obtained in the present study, which can be related to the use of anaerobic fluidized bed reactor (18). The contribution of temperature changes to BOD removal was approximately 42.7%. Additionally, the contribution of aluminum sulfate, sodium alginate, iron chloride, and pectin concentration was 3.8%, 22.4%, 16.3%, and 14.4%, respectively. Therefore, aluminum sulfate concentration was introduced as the least effective factor in BOD reduction.
4. Conclusion
The present study is the first report on the application of fraction of full factorial statistical method and Qualitek-4 software for designing experiments and analyzing laboratory results in order to determine the optimal conditions in terms of flocculation temperature and aluminum sulfate, sodium alginate, iron chloride, and pectin concentrations to achieve the highest percentage of reduction in the organic load of municipal wastewater in Behshahr city (Mazandaran, Iran) and, consequently, reduce the amount of its BOD value. Under optimal conditions, using a combination of natural coagulants such as pectin and sodium alginate and also synthetic coagulants such as aluminum sulfate and iron chloride, as well as controlling the coagulation temperature, BOD can be reduced by up to 34.5% at optimal condition that was achieved at 25°C, using 1, 0.02, 1.5, and 1 g/L ofaluminum sulfate, sodium alginate, iron chloride, and pectin, respectively. Flocculation temperature had the greatest role in BOD reduction and was recognized as the most important factor in the efficiency of flocculation process. The effect of changes in the concentration of sodium alginate was also significant. The changes in aluminum sulfate concentration had the least effect on BOD reduction in the studied wastewater. The main advantage of sodium alginate compared to other studied coagulants is the ability to use the sediments obtained from the coagulation process as feed for livestock and poultry.
Acknowledgments
The authors would like to convey their special thanks to the Vice Chancellor for Research of Islamic Azad University, Qaemshahr Branch.
Conflict of Interests
The authors declare that they have no conflict of interest.
References
- Gadipelly C, Pérez-González A, Yadav GD, Ortiz I, Ibáñez R, Rathod VK. Pharmaceutical industry wastewater: review of the technologies for water treatment and reuse. Ind Eng Chem Res 2014; 53(29):11571-92. doi: 10.1021/ie501210j [Crossref] [ Google Scholar]
- Shokoohi R, Dargahi A, Khamutian R, Vaziri Y. Evaluation of the efficiency of wastewater treatment plants in the removal of common antibiotics from municipal wastewater in Hamadan, Iran. Avicenna J Environ Health Eng 2017; 4(1):10921. doi: 10.5812/ajehe.10921 [Crossref] [ Google Scholar]
- Ardestani F, Abbasi M. Poultry slaughterhouse wastewater treatment using anaerobic fluid bed reactor and aerobic mobile-bed biological reactor. Int J Eng 2019; 32(5):634-40. [ Google Scholar]
- Rezaei H, Rastegar S, Naseri S. Application of chitosan and activated carbon nano-composite in removal of nitrite, phosphate, and ammonia from aquaculture wastewater. Avicenna J Environ Health Eng 2019; 6(2):106-12. doi: 10.34172/ajehe.2019.14 [Crossref] [ Google Scholar]
- Isik Z, Ozbey Unal B, Karagunduz A, Keskinler B, Dizge N. Electrochemical treatment of textile dye bath wastewater using activated carbon cloth electrodes. Avicenna J Environ Health Eng 2020; 7(1):47-52. doi: 10.34172/ajehe.2020.07 [Crossref] [ Google Scholar]
- Shokoohi R, Movahedian H, Dargah A. Evaluation of the efficiency of a biofilter system’s phenol removal from wastewater. Avicenna J Environ Health Eng 2016; 3(1):7449. doi: 10.17795/ajehe-7449 [Crossref] [ Google Scholar]
- Li N, Hu Y, Lu YZ, Zeng RJ, Sheng GP. Multiple response optimization of the coagulation process for upgrading the quality of effluent from municipal wastewater treatment plant. Sci Rep 2016; 6:26115. doi: 10.1038/srep26115 [Crossref] [ Google Scholar]
- Ismail IM, Fawzy AS, Abdel-Monem NM, Mahmoud MH, El-Halwany MA. Combined coagulation flocculation pre treatment unit for municipal wastewater. J Adv Res 2012; 3(4):331-6. doi: 10.1016/j.jare.2011.10.004 [Crossref] [ Google Scholar]
- Ajao V, Bruning H, Rijnaarts H, Temmink H. Natural flocculants from fresh and saline wastewater: comparative properties and flocculation performances. Chem Eng J 2018; 349:622-32. doi: 10.1016/j.cej.2018.05.123 [Crossref] [ Google Scholar]
- Adugna AT, Gebresilasie NM. Aloe steudneri gel as natural flocculant for textile wastewater treatment. Water Pract Technol 2018; 13(3):495-504. doi: 10.2166/wpt.2018.062 [Crossref] [ Google Scholar]
- Cui H, Huang X, Yu Z, Chen P, Cao X. Application progress of enhanced coagulation in water treatment. RSC Adv 2020; 10(34):20231-44. doi: 10.1039/d0ra02979c [Crossref] [ Google Scholar]
- Xiao F, Huang JC, Zhang BJ, Cui CW. Effects of low temperature on coagulation kinetics and floc surface morphology using alum. Desalination 2009; 237(1-3):201-13. doi: 10.1016/j.desal.2007.12.033 [Crossref] [ Google Scholar]
- Smirnova AI, Dyagileva AB. Evaluation of the effect of temperature on the efficiency of wastewater treatment with a composite coagulant-flocculant based on nepheline raw materials. Russ J Appl Chem 2021; 94(2):252-8. doi: 10.1134/S1070427221020166 [Crossref] [ Google Scholar]
- Precious Sibiya N, Rathilal S, Kweinor Tetteh E. Coagulation treatment of wastewater: kinetics and natural coagulant evaluation. Molecules 2021; 26(3):698. doi: 10.3390/molecules26030698 [Crossref] [ Google Scholar]
- Ciciliano JC, Sakurai Y, Myers DR, Fay ME, Hechler B, Meeks S. Resolving the multifaceted mechanisms of the ferric chloride thrombosis model using an interdisciplinary microfluidic approach. Blood 2015; 126(6):817-24. doi: 10.1182/blood-2015-02-628594 [Crossref] [ Google Scholar]
- Czerwionka K, Wilinska A, Tuszynska A. The use of organic coagulants in the primary precipitation process at wastewater treatment plants. Water 2020; 12(6):1650. doi: 10.3390/w12061650 [Crossref] [ Google Scholar]
- Samer M. Biological and chemical wastewater treatment processes. In: Samer M, ed. Wastewater Treatment Engineering. IntechOpen; 2015. p. 40-5. 10.5772/61250.
- Berg KJ. Municipal Wastewater Anaerobic Treatment with Enhanced Clarification [dissertation]. Milwaukee, Wisconsin: Marquette University; 2015. p. 39-43.