Avicenna J Environ Health Eng. 9(1):18-24.
doi: 10.34172/ajehe.2022.03
Original Article
Investigation of the Metal Bioremediation Ability of Two Populations of Peganum Harmala
Kobra Mahdavian 1, * 
Author information:
1Department of Biology, Faculty of Science, Payame Noor University, Tehran 19395-3697, Iran
Abstract
The present study aimed to investigate the effects of zinc exposure (0, 1, 5, 15, 30 mg/L zinc) on the biochemical and physiological parameters of Peganum harmala seedlings. Two populations (metallicolous and non-metallicolous) were compared in Zn tolerance, Zn accumulation, photosynthetic pigments, and enzymatic antioxidant activities. Plants were treated with Zn at concentrations of 0, 1, 5, 15, and 30 mg/L for 14 days. The study results showed that the increase of Zn concentration in the nutrient solution reduced shoot length, root length, root dry weight, shoot dry weight, chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid in both populations; however, the accumulation was more pronounced in metallicolous populations (M) than in non-metallicolous (NM) ones. In response, the activities of antioxidant enzymes such as guaiacol peroxidase, lipoxygenase, superoxide dismutase, catalase, and ascorbate peroxidase were enhanced Zn exposure in both populations. Moreover, it was found that the metallicolous population of P. harmala had a greater capacity to adapt to oxidative stress caused by Zn than the non-metallicolous population, and antioxidative defense in the metallicolous population of P. harmala might have played an essential role in Zn tolerance. Therefore, P. harmala seemed to be a suitable candidate for accumulation; however, it was recommended that further investigations be carried out to explore its metal remediation ability. It is concluded that P. harmala can be a potential candidate for bioremediation of Zn contaminated soils.
Keywords: Accumulation, Tolerance, Peganum harmala L, Antioxidant enzymes
Copyright and License Information
© 2022 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
Please cite this article as follows: Mahdavian K. Investigation of the metal bioremediation ability of two populations of peganum harmala. Avicenna J Environ Health Eng. 2022; 9(1):18-24. doi:10.34172/ajehe.2022.03
1. Introduction
The source of heavy metal contamination is mining and smelting processes, agricultural activities such as pesticides, and urban activities such as heavy metals in fuels, paints, and other materials (1). Mean soil Zn concentrations of 50 and 66 total Zn µg/g soil are typical for mineral and organic soils, respectively, with most agricultural soils containing 10–300 Zn µg/g. Large parts of agricultural soils are contaminated with zinc by biological and anthropological activities, including mining and industrial processes and agricultural practices, such as using Zn fertilizers containing heavy metals (2,3). The excessive amounts of Zn in the soil may cause severe toxicity in plants through inhibiting germination of seeds (4), delayed plant growth (5) and slow root growth (6), induction of foliar chlorosis (7), alteration of mitotic activity (8), changes in membrane integrity and permeability (9) and interference in absorption, transport, and osmotic adjustment of essential ions (10). Zinc accumulates in different parts of the plant and reduces plant growth. Numerous corrective measures must be taken to combat environmental poisoning.
Few plant species, called metallophytes, can survive and reproduce in toxic soils. Zinc hypertolerance is usually limited to the toxic concentrations of metals in the natural environment (11-13). Zinc plays a significant role in regulating the redox balance in the membrane by enhancing the activity of antioxidant enzymes and minimizing the harmful effects of reactive oxygen species (ROS) produced by oxidative stress (14).
Peganum harmala is a perennial glabrous herb that grows in North Africa, the Middle East, and Central Asia (15). P. harmala has been shown to have the ability to accumulate zinc in its shoots and roots in mineral areas (16). Though P. harmala L. is a non-hyperaccumulator species, it has many advantages over hyperaccumulators. Besides, P. harmala has been reported to grow well in the Pb/Zn mining areas, indicating high endurance against the stress of Zn based on its potential for phytoremediation. Currently, there are no reports about the effect of Zn on the biochemical and physiological parameters of P. harmala. Therefore, the present study tried to investigate the effect of Zn on P. harmala seedlings at different concentrations. The populations were compared in Zn tolerance, Zn accumulation, photosynthetic pigments, and enzymatic antioxidant activities.
2. Materials and Methods
2.1. Plant Materials and Zn Treatments
Peganum Harmala seeds were collected from more than 40 plants at the mining site of Koshk (metallicolous) and non-contaminated areas of Kerman (non-metallicolous), both in Iran. The amounts of soil metal and pH at these sites are given by Mahdavian et al (17).
For seed sowing, 9 cm diameter plastic pots containing a mixture of fine and coarse perlite were prepared. Six P. harmala seeds were planted in each pot, and three replications for each concentration were used. After five days of distilled water, the seedlings were irrigated with 0.5 Hoagland nutrient solution for 40 days (18). After 45 days, the plants were exposed to Zn (concentrations of 0, 1, 5, 15, 30 ppm ZnSo4) for two weeks. The pH of the nutrient solution and the nutrient solution containing Zn was adjusted to values ranging from 0.5 to 6.5. The nutrient solutions were replaced with fresh solutions every week, and the plants were grown in a culture chamber with alternating night/day temperatures of 25/20°C with 16 hours of light.
2.2. Biomass and Zn Concentration Measurement
In order to determine and measure the amount of accumulated metal in the roots and aerial parts of the plant, 0.05-0.1 g of the plants were dried, crushed, and placed in glass tubes. Then, 4 mL of hydrochloric acid (37%), 4 mL of nitric acid (65%), 1.5 mL of perchloric acid, and 1.5 mL of hydrogen peroxide were added to each sample. Afterward, samples were placed in the sand bath at 150-200°C for 2 hours. Zinc was measured using a flame atomic absorption spectrophotometer, as described in a study by Reeves et al (19).
2.3. Chlorophyll and Carotenoids
Fresh leaf tissues were ground with a mortar and pestle under liquid N2. Eighty percent (v/v) acetone was added to extract pigments, and after centrifugation of the supernatant for 10 minutes at 10 000 rpm, optical density (OD) was measured at 470, 646.8, and 663.2 nm byusing UV-Vis spectrophotometer. Extinction coefficients and equations reported by Lichtenthaler were used to calculate the amounts of chlorophyll a, chlorophyll b, andcarotenoids (20).
2.4. Enzyme Extraction and Assays
Briefly, 0.1 g of leaf sample was homogenized in 6 mL of 50 mM potassium phosphate buffer (pH 7.8) containing 0.2 mM EDTA and 2% (w/v) polyvinylpyrrolidone (PVP) in an ice bath. The homogenate was centrifuged for 20 minutes at 12 000 rpm at 4°C, and the supernatant was used to measure enzyme activities.
Superoxide dismutase activity was determined based on the method described by Giannopolitis and Ries (21). Lipoxygenase activity was measured according to the method proposed by Doderer et al (22). Guaiacol peroxidase activity was measured by Plewa et al (23). Catalase activity was measured according to the method used by Aebi (24), and ascorbate peroxidase activity was measured according to Nakano and Asada (25) and Boominathan and Doran (26).
2.5. Statistical Analysis
The data were analyzed by two-way ANOVA, with Zn exposure concentration and population as fixed factors. The means were compared using the Tukey’s test. P < 0.05 was considered statistically significant.
3. Results and Discussion
Decreases in the dry weights of roots and shoots were observed as a result of different Zn concentrations, according to Fig. 1. The lowest dry weights of roots and shoots treated with 30 mg/L of Zn in non-metallicolous populations were 35% and 37%, respectively. There was no significant difference between the two populations at different concentrations. Furthermore, the analysis of variance showed that Zn concentration x population interaction was insignificant for the shoot and root dry weight. Fig. 2 shows that Zn treatment significantly reduced shoot and root length compared to the control plans in both populations. In terms of shoot length, there was a significant difference at different concentrations in each population, so the lowest shoot length was observed at 30 mg/L concentration in both populations compared to control plants. Furthermore, the analysis of variance showed that the Zn concentration x population interaction was insignificant for the shoot and root length.
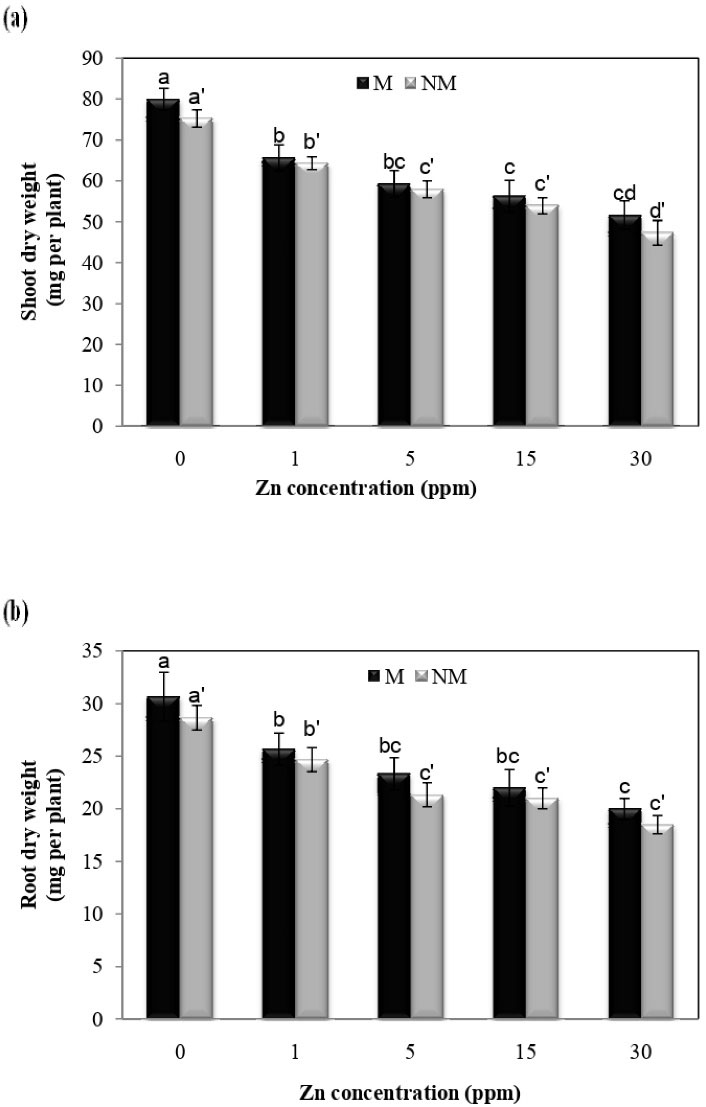
Figure 1.
Effect of Zn on the Dry Weight of Shoots and Roots in Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
.
Effect of Zn on the Dry Weight of Shoots and Roots in Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
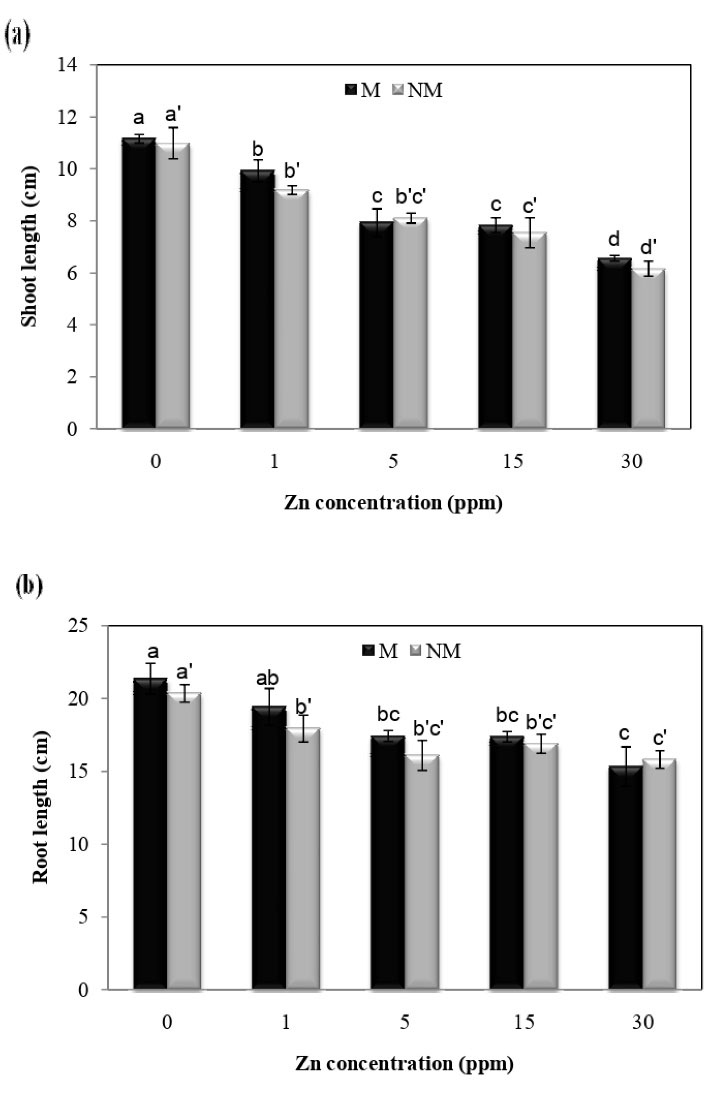
Figure 2.
Effect of Zn on the Length of Shoots and Roots in Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after to Increasing Zn Concentrations (mg/L) for 2 Weeks.
.
Effect of Zn on the Length of Shoots and Roots in Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after to Increasing Zn Concentrations (mg/L) for 2 Weeks.
The concentration of Zn in the shoots and roots significantly increased compared to control plants in both populations at all treatment concentrations. The highest Zn concentration in shoots was observed at 30 mg/L in both populations (Figs. 3a and b). Furthermore, the analysis of variance showed that the Zn concentration x population interaction was insignificant on the accumulation of Zn in shoots and roots.
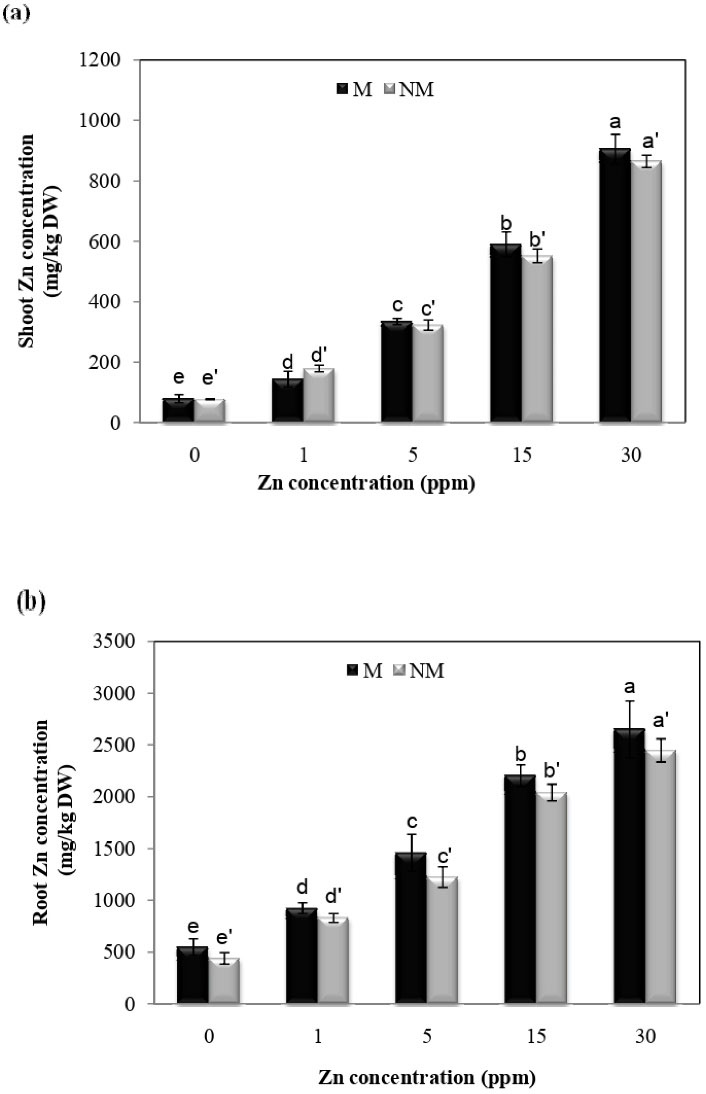
Figure 3.
Concentration (mg/kg DW) of Zn in Shoots and Roots in Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) After Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
.
Concentration (mg/kg DW) of Zn in Shoots and Roots in Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) After Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
Despite the structural role of Zn in some enzymes and metabolic processes, the excess accumulation of this metal in the soil causes poisoning and lack of plant growth (8,27). Growth inhibition is one of the responses of plants to metal toxicity (28,29). Disturbance in cell division and, consequently, prolonging plant growth can justify biomass reduction in the presence of these metals (28,30). This study showed that the longitudinal growth of the root of P. harmala decreased at high concentrations of Zn in comparison with the control plants. It was reported that Zn exposure caused a significant reduction in the growth of Cajanus cajan roots, which is consistent with this study (8). Studies have shown that excessive Zn decreases root growth by decreasing the number of cells in the meristem areas (31). However, reduced root growth due to zinc toxicity impairs the uptake and transport of essential nutrients in the plant (8,31). It has been reported that the excess accumulation of Zn in the roots and aerial parts of the plant interfered with the translocation of iron. This decrease in the iron content of the organs is associated with metabolic changes and reduced plant growth in Zn-contaminated areas (32,33). Furthermore, in some reports, inhibition of plant growth due to Zn toxicity may be due to the competition of this metal with the absorption and transfer of phosphorus in the plant. As shown in this study, Zn inhibited the growth of the shoots and roots and reduced biomass in the P. harmala. Another reason for reduced growth and biomass due to zinc toxicity is a change in the metabolic status of the plant (8). In this study, the dry weight of shoots and roots in both populations decreased with increasing Zn content. Although both populations showed very similar degrees of growth inhibition at all of Zn exposure levels tested, there was no significant difference between the two populations at different concentrations. Festuca ovina and Matthiola flavida are facultative metallophytes from Iran which seem capable of the considerable foliar Zn accumulation in nature (18,34). Liu et al (35) reported that the dry weight of Phyllostachys pubescens decreased by Zn treatment.
Most soil samples of Koshk mine have neutral to alkaline pH, which can be the reason for the insignificance of Zn tolerance in the local P. harmala population (16). However, both populations displayed growth inhibition due to Zn exposure; Zn concentration was higher in the shoots and roots in the metallicolous population than in the non-metallicolous one. Result clearly shows that the metallicolous population is more tolerant of Zn toxicity than the non-metallicolous one.
Changes in chlorophyll-a concentration were also observed at different Zn concentrations, according to Fig. 4a. The lowest leaf chlorophyll, a content at 30 mg/L in metallicolous and non-metallicolous populations, was 32.2% and 29.5%, respectively. There was no significant difference between the two populations at different concentrations. Moreover, the results of ANOVA showed that interaction between population and treatment had no significant effect on chlorophyll-a concentration.
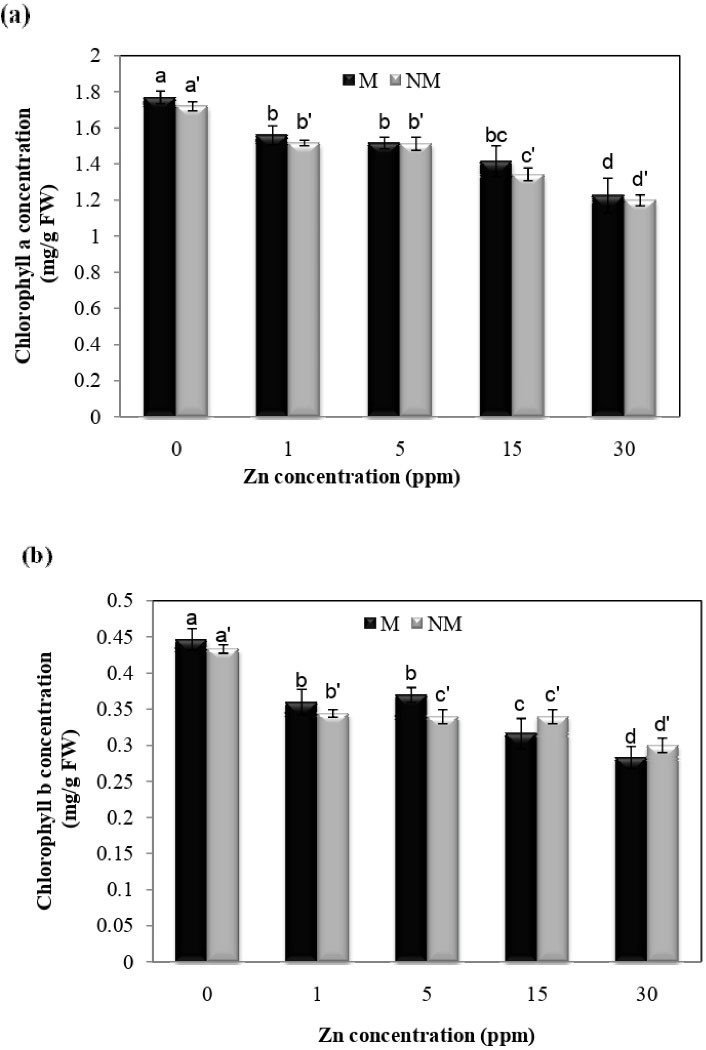
Figure 4.
Effect of Zn on the Leaf Concentration of Chlorophyll a (a) and Chlorophyll b (b) in Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
.
Effect of Zn on the Leaf Concentration of Chlorophyll a (a) and Chlorophyll b (b) in Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
Based on Fig. 4b, chlorophyll b levels showed a significant decrease at different treatment concentrations compared to control plants in both populations. The highest amount of chlorophyll b in control plants and the lowest amount of chlorophyll b at 30 mg/L in metallicolous and non-metallicolous were 36.4 and 30.3%, respectively. The results of ANOVA showed that the interaction between population and treatment had a significant effect on chlorophyll concentration (P < 0.01).
According to Fig. 5, it is known that Zn treatment at all concentrations significantly reduced carotenoid concentration in both populations of P. harmala compared to the control plants. There is no significant difference between the two populations. Besides, the results of ANOVA showed that interaction between population and treatment had no significant effect on carotenoid content.
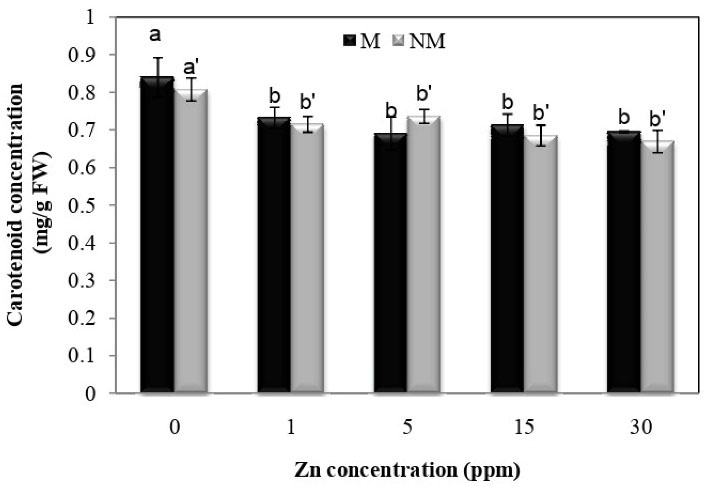
Figure 5.
Effect of Zn on the Leaf Concentration of Carotenoid in Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
.
Effect of Zn on the Leaf Concentration of Carotenoid in Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
The effects of Zn on the content of chlorophyll and carotenoids are different depending on the plant species and the concentration of the metal used. By protecting the sulfhydryl group (-SH), Zn causes chlorophyll synthesis. The precursor to chlorophyll synthesis is porphobilinogen, which is required to form Zn and magnesium (36). Finally, the formation and completion of chlorophyll are facilitated (37,38).
Sagardoy et al (39) observed a decrease in the concentration of photosynthetic pigments in sugar beet plants in response to Zn. A decrease in chlorophyll content has also been reported in tomatoes under Zn stress (10). These reports are consistent with the results of this study.
According to Fig. 6, it is determined that treatment at all concentrations significantly increased catalase activity compared to control plants in both populations. There was also a significant difference between the two populations according to the analysis; the highest activity of catalase at a concentration of 30 mg/L was observed in the metal population compared to other concentrations with an increase of 245.3% compared to control plants. Analysis of variance showed that the interaction between population and Zn treatment on catalase activity was significant (P < 0.01).
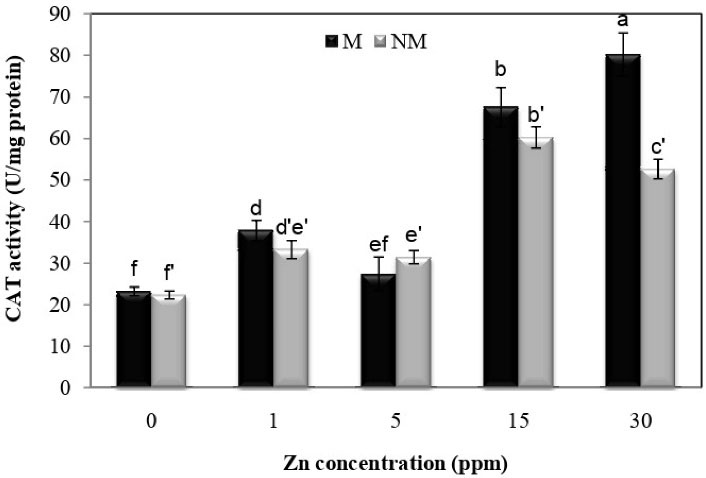
Figure 6.
Changes in CAT Activities in the Leaves of Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
.
Changes in CAT Activities in the Leaves of Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
Fig. 7 showed that Zn treatment at all concentrations significantly increased guaiacol peroxidase (GPX) activity compared to control plants in both populations. There was also a significant difference between the two populations at concentrations of 15 and 30 mg/L. The metal population had the maximum activity of guaiacol at a concentration of 15 mg/L compared to other concentrations, with a 257.1% increase over the control group. The interaction between population and Zn treatment on GPX activity was significant (P < 0.01), according to the analysis of variance.
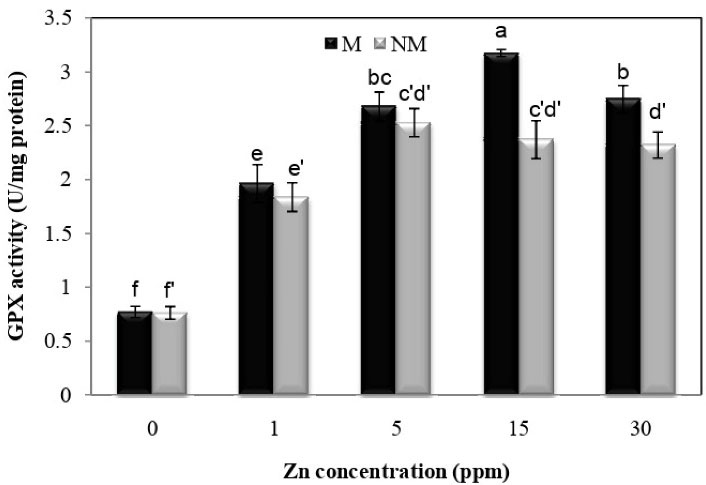
Figure 7.
Changes in GPX Activities in the Leaves of Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
.
Changes in GPX Activities in the Leaves of Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
According to Fig. 8, Zn treatment at all concentrations significantly increased ascorbate peroxidase activity compared to control plants in both populations. There was also a significant difference between the two populations at concentrations of 15 and 30 mg/L. The metal population had the maximum activity of ascorbate peroxidase at a concentration of 30 mg/L compared to other concentrations, with a 330.3% percent increase over the control group. The interaction between population and Zn treatment on ascorbate peroxidase activity was significant (P < 0.01), according to the analysis of variance.
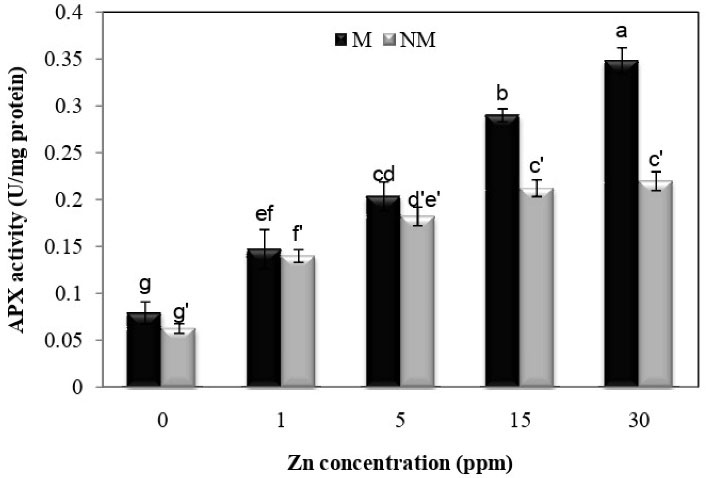
Figure 8.
Changes in APX Activities in the Leaves of Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
.
Changes in APX Activities in the Leaves of Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
According to Fig. 9, an increase in Zn concentration significantly increased lipoxygenase activity compared to the controls in both populations. The highest lipoxygenase activity was observed at 30 mg/L concentration of Zn in both populations. There was also no significant difference between the two populations. Furthermore, the analysis of variance showed that the Zn concentration x population interaction was insignificant for lipoxygenase activity.
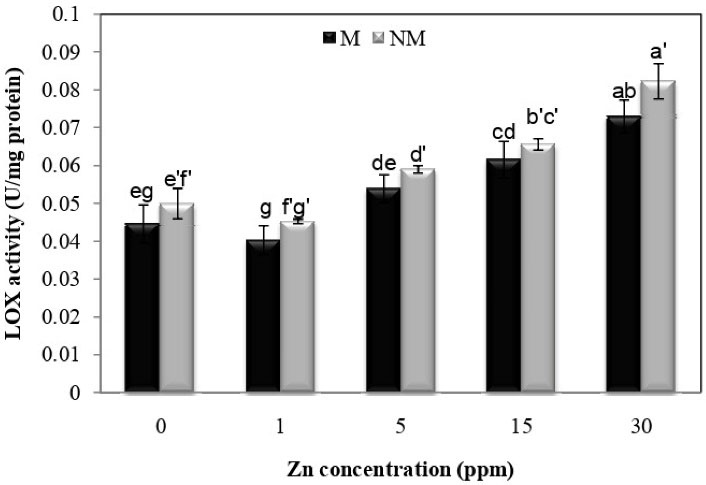
Figure 9.
Changes in LOX Activities in the Leaves of Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
.
Changes in LOX Activities in the Leaves of Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
As shown in Fig. 10, there was a significant difference in superoxide dismutase activity at different concentrations of Zn in each population. There was a significant difference between the two populations at the concentration of 30 mg/L. The metal population had the maximum activity of superoxide dismutase at a concentration of 30 mg/L compared to other concentrations, with a 86.4% percent increase over the control group. The interaction between population and Zn treatment on superoxide dismutase activity was significant (P < 0.01), according to the analysis of variance.
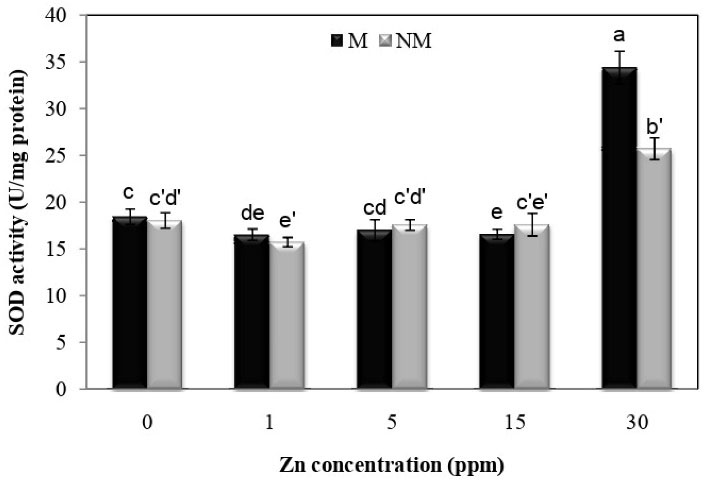
Figure 10.
hanges in SOD Activities in the Leaves of Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
.
hanges in SOD Activities in the Leaves of Two Populations of P. harmala (M: metallicolous population; NM: non-metallicolous population) after Exposure to Increasing Zn Concentrations (mg/L) for 2 Weeks.
Antioxidant enzymes form a protective enzyme system against metal-induced oxidative stress (40,41). The results of the present study showed a significant increase in the activity of the antioxidant enzyme under Zn stress compared to the control plants. Among the defense mechanisms, antioxidant enzymes are the most effective system for scavenging ROS (41,42). Superoxide dismutase acts against superoxide radicals produced in different cellular segments (43). In this study, Zn stress significantly increased superoxide dismutase activity in P. harmala. This study’s results are consistent with the findings of Galal’s study (44) on other plants. Catalase is a metalloenzyme involved in the plant’s defense against the oxidative stress (45). Catalase activity in Kandelia obovata increased under Zn stress. Our results are consistent with the response of catalase to excess Zn in Vitis vinifera (46). Zinc is required for catalase activity involved in the detoxification of H2O2 (47). Zn counteracts the harmful effects of ROS by enhancing the activity of antioxidant enzymes (48). Ascorbate peroxidase, an antioxidant enzyme, plays a vital role in removing H2O2 (49). In this study, ascorbate peroxidase activity was enhanced under Zn stress. These results are consistent with a study conducted by Amiri et al (14), who reported that ascorbate peroxidase activity enhanced in response to Zn stress. This high tolerance to Zn accumulation in the plant may be well developed at the mining site of Koshk. Since P. harmala is not a Zn hyperaccumulator, the metallicolous population is expected to adopt an excluder strategy for tolerance. Therefore, in this study, the metallicolous population has inherited a relatively high level of Zn accumulation from an ancestral non-metallicolous population. In this case, the metallicolous population from Koshk showed more tolerance to Zn exposure in the area. To clarify this, further studies are needed on many more metallicolous and non-metallicolous populations.
4. Conclusion
Based on growth responses and antioxidant enzyme activity, it can be concluded that the metallicolous population of P. harmala demonstrated higher tolerance to Zn than the non-metallicolous population. The highest enzyme activity was observed at the concentration of 30 mg/L in the metallicolous population compared to the other concentrations. Both populations were evenly tolerant of Zn, but the metallicolous plants exhibited high levels of Zn accumulation in both roots and shoots. Considering the above results, Peganum harmala could be used for bioremediation of Zn contaminated soils.
Acknowledgments
The author would like to thank Payame Noor University Research Council for approval and provide financial support.
Conflict of Interests
The authors declare that there is no conflict of interest.
References
- Lone MI, He ZL, Stoffella PJ, Yang XE. Phytoremediation of heavy metal polluted soils and water: progresses and perspectives. J Zhejiang Univ Sci B 2008; 9(3):210-20. doi: 10.1631/jzus.B0710633 [Crossref] [ Google Scholar]
- Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK. Transgenic tobacco overexpressing glyoxalase pathway enzymes grow and set viable seeds in zinc-spiked soils. Plant Physiol 2006; 140(2):613-23. doi: 10.1104/pp.105.073734 [Crossref] [ Google Scholar]
- Dudka S, Piotrowska M, Terelak H. Transfer of cadmium, lead, and zinc from industrially contaminated soil to crop plants: a field study. Environ Pollut 1996; 94(2):181-8. doi: 10.1016/s0269-7491(96)00069-3 [Crossref] [ Google Scholar]
- Michael PI, Krishnaswamy M. The effect of zinc stress combined with high irradiance stress on membrane damage and antioxidative response in bean seedlings. Environ Exp Bot 2011; 74:171-7. doi: 10.1016/j.envexpbot.2011.05.016 [Crossref] [ Google Scholar]
- Andrade SA, Gratão PL, Schiavinato MA, Silveira AP, Azevedo RA, Mazzafera P. Zn uptake, physiological response and stress attenuation in mycorrhizal jack bean growing in soil with increasing Zn concentrations. Chemosphere 2009; 75(10):1363-70. doi: 10.1016/j.chemosphere.2009.02.008 [Crossref] [ Google Scholar]
- Lingua G, Franchin C, Todeschini V, Castiglione S, Biondi S, Burlando B. Arbuscular mycorrhizal fungi differentially affect the response to high zinc concentrations of two registered poplar clones. Environ Pollut 2008; 153(1):137-47. doi: 10.1016/j.envpol.2007.07.012 [Crossref] [ Google Scholar]
- Wang C, Zhang SH, Wang PF, Hou J, Zhang WJ, Li W. The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere 2009; 75(11):1468-76. doi: 10.1016/j.chemosphere.2009.02.033 [Crossref] [ Google Scholar]
- Rout GR, Das P. Effect of metal toxicity on plant growth and metabolism: I Zinc. Agronomie 2003; 23(1):3-11. [ Google Scholar]
- Stoyanova Z, Doncheva S. The effect of zinc supply and succinate treatment on plant growth and mineral uptake in pea plant. Braz J Plant Physiol 2002; 14(2):111-6. doi: 10.1590/s1677-04202002000200005 [Crossref] [ Google Scholar]
- Cherif J, Derbel N, Nakkach M, Bergmann H, Jemal F, Lakhdar ZB. Analysis of in vivo chlorophyll fluorescence spectra to monitor physiological state of tomato plants growing under zinc stress. J Photochem Photobiol B 2010; 101(3):332-9. doi: 10.1016/j.jphotobiol.2010.08.005 [Crossref] [ Google Scholar]
- Antonovics J, Bradshaw AD, Turner RG. Heavy metal tolerance in plants. Adv Ecol Res 1971; 7:1-85. [ Google Scholar]
- Macnair MR. The genetics of metal tolerance in vascular plants. New Phytol 1993; 124(4):541-59. doi: 10.1111/j.1469-8137.1993.tb03846.x [Crossref] [ Google Scholar]
- Schat H, Vooijs R, Kuiper E. Identical major gene loci for heavy metal tolerances that have independently evolved in different local populations and subspecies of Silene vulgaris. Evolution 1996; 50(5):1888-95. doi: 10.1111/j.1558-5646.1996.tb03576.x [Crossref] [ Google Scholar]
- Amiri A, Baninasab B, Ghobadi C, Khoshgoftarmanesh AH. Zinc soil application enhances photosynthetic capacity and antioxidant enzyme activities in almond seedlings affected by salinity stress. Photosynthetica 2016; 54(2):267-74. doi: 10.1007/s11099-016-0078-0 [Crossref] [ Google Scholar]
- Shamsa F, Monsef HR, Ghamooghi R, Verdian-Rizi M. Spectrophotometric determination of total alkaloids in Peganum harmala L using bromocresol green. Res J Phytochem 2007; 1(2):79-82. doi: 10.3923/rjphyto.2007.79.82 [Crossref] [ Google Scholar]
- Mahdavian K, Ghaderian SM, Torkzadeh-Mahani M. Accumulation and phytoremediation of Pb, Zn, and Ag by plants growing on Koshk lead–zinc mining area, Iran. J Soils Sediments 2017; 17(5):1310-20. doi: 10.1007/s11368-015-1260-x [Crossref] [ Google Scholar]
- Mahdavian K, Ghaderian SM, Schat H. Pb accumulation, Pb tolerance, antioxidants, thiols, and organic acids in metallicolous and non-metallicolous Peganum harmala L under Pb exposure. Environ Exp Bot 2016; 126:21-31. doi: 10.1016/j.envexpbot.2016.01.010 [Crossref] [ Google Scholar]
- Mohtadi A, Ghaderian SM, Schat H. A comparison of lead accumulation and tolerance among heavy metal hyperaccumulating and non-hyperaccumulating metallophytes. Plant Soil 2012; 352(1):267-76. doi: 10.1007/s11104-011-0994-5 [Crossref] [ Google Scholar]
- Reeves RD, Baker AJM, Borhidi A, Berazaín R. Nickel hyperaccumulation in the serpentine flora of Cuba. Ann Bot 1999; 83(1):29-38. doi: 10.1006/anbo.1998.0786 [Crossref] [ Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 1987; 148:350-82. [ Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutases: I Occurrence in higher plants. Plant Physiol 1977; 59(2):309-14. doi: 10.1104/pp.59.2.309 [Crossref] [ Google Scholar]
- Doderer A, Kokkelink I, van der Veen S, Valk BE, Schram AW, Douma AC. Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim Biophys Acta 1992; 1120(1):97-104. doi: 10.1016/0167-4838(92)90429-h [Crossref] [ Google Scholar]
- Plewa MJ, Smith SR, Wagner ED. Diethyldithiocarbamate suppresses the plant activation of aromatic amines into mutagens by inhibiting tobacco cell peroxidase. Mutat Res 1991; 247(1):57-64. doi: 10.1016/0027-5107(91)90033-k [Crossref] [ Google Scholar]
- Aebi H. Catalase. In: Bergmeyer HU, ed. Methods of Enzymatic Analysis. Vol III. 3rd ed. Weinheim, Germany: Verlage Chemie; 1983. p. 273-86.
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 1981; 22(5):867-80. [ Google Scholar]
- Boominathan R, Doran PM. Ni-induced oxidative stress in roots of the Ni hyperaccumulator, Alyssum bertolonii. New Phytol 2002; 156(2):205-15. doi: 10.1046/j.1469-8137.2002.00506.x [Crossref] [ Google Scholar]
- Lagua JC. Performance of transplanted sweet corn fertilized with zinc sulphate. J Crit Rev 2020; 7:5103-9. [ Google Scholar]
- Bonnet M, Camares O, Veisseire P. Effects of zinc and influence of Acremonium lolii on growth parameters, chlorophyll a fluorescence and antioxidant enzyme activities of ryegrass (Lolium perenne L cv Apollo). J Exp Bot 2000; 51(346):945-53. [ Google Scholar]
- Mahdavian K. Effect of citric acid on antioxidant activity of garden cress (Lepidium sativum L) under chromium stress. Journal of Plant Process and Function 2021; 9(40):1-12. [ Google Scholar]
- Mahdavian K. Effect of citric acid on antioxidant activity of red bean (Phaseolus calcaratus L) under Cr + 6 stress. S Afr J Bot 2021; 139:83-91. doi: 10.1016/j.sajb.2021.02.002 [Crossref] [ Google Scholar]
- Jain R, Srivastava S, Solomon S, Shrivastava AK, Chandra A. Impact of excess zinc on growth parameters, cell division, nutrient accumulation, photosynthetic pigments and oxidative stress of sugarcane (Saccharum spp). Acta Physiol Plant 2010; 32(5):979-86. doi: 10.1007/s11738-010-0487-9 [Crossref] [ Google Scholar]
- Rosen JA, Pike CS, Golden ML. Zinc, iron, and chlorophyll metabolism in zinc-toxic corn. Plant Physiol 1977; 59(6):1085-7. doi: 10.1104/pp.59.6.1085 [Crossref] [ Google Scholar]
- Ambler JE, Brown JC, Gauch HG. Effect of zinc on translocation of iron in soybean plants. Plant Physiol 1970; 46(2):320-3. doi: 10.1104/pp.46.2.320 [Crossref] [ Google Scholar]
- Brown G, Brinkmann K. Heavy metal tolerance in Festuca ovina L from contaminated sites in the Eifel Mountains, Germany. Plant Soil 1992; 143(2):239-47. [ Google Scholar]
- Liu D, Chen J, Mahmood Q, Li S, Wu J, Ye Z. Effect of Zn toxicity on root morphology, ultrastructure, and the ability to accumulate Zn in Moso bamboo (Phyllostachys pubescens). Environ Sci Pollut Res Int 2014; 21(23):13615-24. doi: 10.1007/s11356-014-3271-3 [Crossref] [ Google Scholar]
- Beale SI. Enzymes of chlorophyll biosynthesis. Photosynth Res 1999; 60(1):43-73. doi: 10.1023/a:1006297731456 [Crossref] [ Google Scholar]
- Lebedev N, Timko MP. Protochlorophyllide photoreduction. Photosynth Res 1998; 58(1):5-23. doi: 10.1023/A:1006082119102 [Crossref] [ Google Scholar]
- Stobart AK, Griffiths WT, Ameen-Bukhari I, Sherwood RP. The effect of Cd2 + on the biosynthesis of chlorophyll in leaves of barley. Physiol Plant 1985; 63(3):293-8. doi: 10.1111/j.1399-3054.1985.tb04268.x [Crossref] [ Google Scholar]
- Sagardoy R, Morales F, López-Millán AF, Abadía A, Abadía J. Effects of zinc toxicity on sugar beet (Beta vulgaris L) plants grown in hydroponics. Plant Biol (Stuttg) 2009; 11(3):339-50. doi: 10.1111/j.1438-8677.2008.00153.x [Crossref] [ Google Scholar]
- Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 1984; 219(1):1-14. doi: 10.1042/bj2190001 [Crossref] [ Google Scholar]
- Liu K, Li C, Tang S, Shang G, Yu F, Li Y. Heavy metal concentration, potential ecological risk assessment and enzyme activity in soils affected by a lead-zinc tailing spill in Guangxi, China. Chemosphere 2020; 251:126415. doi: 10.1016/j.chemosphere.2020.126415 [Crossref] [ Google Scholar]
- Abbaspour H. Effect of salt stress on lipid peroxidation, antioxidative enzymes, and proline accumulation in pistachio plants. J Med Plants Res 2012; 6(3):526-9. [ Google Scholar]
- Sharaf AEM, Sofy AR, Sofy MR, Mabrouk AS. Effect of zinc on antioxidant enzyme activities of common bean under drought stress. J Biol Chem Res 2016; 33(2):669-76. [ Google Scholar]
- Galal A. Exogenous application of zinc mitigates the deleterious effects in eggplant grown under salinity stress. J Plant Nutr 2019; 42(8):915-27. doi: 10.1080/01904167.2019.1584221 [Crossref] [ Google Scholar]
- Luo ZB, He XJ, Chen L, Tang L, Gao SH, Chen FA. Effects of zinc on growth and antioxidant responses in Jatropha curcas seedlings. Int J Agric Biol 2010; 12(1):119-24. [ Google Scholar]
- Yang Y, Sun C, Yao Y, Zhang Y, Achal V. Growth and physiological responses of grape (Vitis vinifera “Combier”) to excess zinc. Acta Physiol Plant 2011; 33(4):1483-91. doi: 10.1007/s11738-010-0687-3 [Crossref] [ Google Scholar]
- Wani AS, Ahmad A, Hayat S, Fariduddin Q. Salt-induced modulation in growth, photosynthesis and antioxidant system in two varieties of Brassica juncea. Saudi J Biol Sci 2013; 20(2):183-93. doi: 10.1016/j.sjbs.2013.01.006 [Crossref] [ Google Scholar]
- Weisany W, Sohrabi Y, Heidari G, Siosemardeh A, Ghassemi-Golezani K. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L). Plant Omics 2012; 5(2):60-7. [ Google Scholar]
- Najami N, Janda T, Barriah W, Kayam G, Tal M, Guy M. Ascorbate peroxidase gene family in tomato: its identification and characterization. Mol Genet Genomics 2008; 279(2):171-82. doi: 10.1007/s00438-007-0305-2 [Crossref] [ Google Scholar]