Avicenna J Environ Health Eng. 7(2):78-85.
doi: 10.34172/ajehe.2020.12
Original Article
Relationship Between Trace Element Content in the Brain of Bony Fish Species and Their Food Items in the Southwest of the Caspian Sea Due to Anthropogenic Activities
Masoud Sattari 1, 2, *  , Mohammad Forouhar Vajargah 1, Mehdi Bibak 1, Shima Bakhshalizadeh 2
, Mohammad Forouhar Vajargah 1, Mehdi Bibak 1, Shima Bakhshalizadeh 2
Author information:
1Department of Fisheries, Faculty of Natural Resources, University of Guilan, Sowmeh Sara, Iran
2Department of Marine Sciences, Caspian Sea Basin Research Center, University of Guilan, Rasht, Iran
Abstract
The trace elements (TEs) in aquatic environments due to anthropogenic activities are readily available to aquatic organisms. There are 153 fish species inhabiting the Caspian Sea. However, little is known about TE (TE) concentrations in these species. In this study, we investigated the effects of TEs levels in the brain of some bony fish species inhabiting the Caspian basin. Totally, 405 fish from nine species with different feeding behaviors including Leuciscus aspius (n = 20) and Perca fluviatilis (n = 37) as piscivores, Rutilus kutum (n = 27) and Rutilus caspius (n = 71) as carnivores, Vimba persa (n = 56), Ponticola caspia (n = 25) and Tinca tinca (n = 31) as mollusivores, and Alburnus chalcoides (n = 77) and Alosa braschnikowi (n = 61) as zooplanktivores were collected from the southwest of the Caspian Sea basin from September 2017 to June 2018. The ICP-OES was used to measure TEs in the brain tissue of fish. The variability of TEs concentrations in the brain tissues of these fishes by principal component analysis (PCA) was decreased to 63.59% and 17.68% for PC1 and PC2, respectively, exhibiting that 81.27% of the total variability is associated with K, Mg, P, S, Zn, and Al. The two-dimensional diagrams showed the weight of each component in PCA. The PC1 was mostly influenced by P, Mg, K, and Zn, while the greatest value in PC2 belonged to Al. Furthermore, the entire number of elements determined in this study was found to be a suitable indicator for the distinction between fish species based on their feeding items.
Keywords: Fish, Caspian Sea basin, Trace elements, Bony fish, Feeding behavior
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
The role of nutritional behavior in mental activity, especially neuroscience is an apparent inter-disciplinary field of investigation seeking to understand the impact of feeding items on brain health across the lifespan (1). Research in this field has recently prospered and illustrated aspects of food from entire food items to specific elements affecting brain function and structure, and so have important roles in understanding the life history events (2). The influences of dietary elements on the neuronal function that are responsible for the action of food items on the brain have been proved (3). However, the diet has traditionally been classified as a means to provide the body with energy and building material. The role of micronutrients in the function of the brain has received the consideration of many researchers over the history of nutrition science (4). Some TEs including Zn, Mn, and Co are necessary for the function and growth of the human brain, while some others such as Hg and Pb create adverse effects on them (3,4), although these effects have not yet been studied in fish.
On the other hand, for estimating the risk of environmental pollutants in the aquatic organism, trace element (TE) accumulations via ingestion, adsorption, and respiration should be assessed (5,6). The TEs accumulations are elevated by anthropogenic activities in marine environments (7,8). TEs can be classified as toxic (nickel, mercury, cadmium, arsenic, lead, etc.), necessary including cobalt and vanadium and also essential such as iron, manganese, copper, zinc, and selenium (6,9). Aquatic organisms such as fish are assessed as an appropriate index for the long-term accumulation of TEs in the aquatic environments. Thus, numerous authors have examined TEs accumulation in various fishes (8).
The Caspian Sea basin as the largest lake of the world has characteristics common to both seas and lakes but is not a freshwater one (10-12). It is bordered by the Republic of Azerbaijan, Russia (Astrakhan Oblast, Dagestan, Kalmykia), Turkmenistan, Iran (Mazandaran, Guilan, and Golestan provinces), and Kazakhstan. It is highly polluted due to growing urbanization, agricultural production, and industrial activities in most riparian countries around the basin (13).
Various studies were carried out on TE contaminations in aquatic resources of Iran including those on inshore sediments (14), on two sturgeon species, i.e., Acipenser stellatusand Acipenser persicus(15) and also on Mugil auratus in the southern part of the Caspian Sea (16). Other studies were also carried out by Abtahi et al (17) on Liza aurataand by Ebrahimzadeh et al (18) on Liza saliens from the southern part of this sea. Additionally, another study was performed on Liza abuobtained from the Karoon and Karkheh watershed (19,20). Furthermore, Alipour and Banagar (21) examined some fish collected from the southeast Caspian basin in the Gorgan bay and also Silurus glanisfrom Anzali lagoon (22). TEs levels were also investigated in the liver and muscle tissues of Tinca tincaand Perca fluviatilisin Anzali Wetland (23) as well as R. kutumby Mirzajani et al (24) but there is no study on the brain of fish from this basin. Therefore, the main objective of this investigation is to distinguish the concentrations of some TEs in the brain tissue of fish from the southwest of the Caspian basin and compare these levels in the brain tissue of fish with different food items.
2. Materials and Methods
This experiment was carried out at three fisheries, including Anzali, Kiashahr, and Astara in the south-west of the Caspian Sea basin (Fig. 1). Samples were obtained from September 2017 to January 2018, and totally 405 fish from nine species with different feeding behaviors were sampled including Leuciscus aspius (n = 20), and Perca fluviatilis (n = 37) as piscivores, Rutilus kutum (n = 27) and Rutilus caspius(n = 71) as carnivores, Vimba persa (n = 56), Ponticola caspia (n = 25) and Tinca tinca(n = 31) as molluscivores, and Alburnus chalcoides (n = 77) and Alosa braschnikowi (N = 61) as zooplanktivores. The samples were transferred to the laboratory using a cooler box at 4°C. The specimens were washed with pure water, the brain of samples was separated and oven-dried at 80°C for 18 hours (8). Ten millilitres of 65% nitric acid was used to digest 0.5 g of each fish tissue in a microwave and to extract TEs. Digested tissues were filtered through Whatman filter paper number 40 and finally were diluted with pure water to reach the exact volume. To determine the concentration of TEs in the specimens, ICP-OES (Zarazma Co. Tehran, Iran) was applied with megapixel charge coupled device detector arrays, facilitating simultaneous monitoring of TEs emission. To check the precision of the analytical method, a multi-element standard solution was applied for the calibration. Standard stock solutions of TEs at the concentration of 1000 mg L-1 were used to produce the solutions with suitable dilution. Standard liquids were of analytical grade (Merck, Darmstadt, Germany). Distilled deionized water was applied in all dilution procedures. Instrument detection limits were 0.1 mg kg-1 for major elements such as Fe and Al, and 0.02 mg kg-1 for TEs.
Figure 1.
Fish Sampling Sites in the Southwest of the Inshore Water of Caspian Basin.
.
Fish Sampling Sites in the Southwest of the Inshore Water of Caspian Basin.
2.1. Statistical Analysis
The TEs of the brain were examined for the homogeneity of variances and normality of the data. Then, one-way analysis of variances was performed to assess possible variability of TEs concentrations. Tukey’s test was used to compare the differences between means. The Kruskal-Wallis test was employed for non-parametric cases (25). Principal component analysis (PCA) was applied for reduction of the number of variables without any data missing (26). The values of cumulative variances and also the number of principal components against eigenvalues were given to determine the important elements and principal components. The exact place of each specimen was determined by discriminant function analysis (DFA). Based on mean linkage method, cluster dendrogram using Euclidian distance was drawn by Ward’s approach. This method was performed just as a complementary procedure to DFA. All statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL). α = 0.05 was considered statistically significant.
3. Results and Discussion
In general, human activities such as the establishment of factories, industrial centers, residential houses and the existence of commercial and fishing ports cause the entry of heavy metals into aquatic environments (27-29). The use of metal tools such as the hulls of commercial and fishing vessels and the entry of agricultural effluents containing pesticides are only a small part of human destructive activities in the environment, especially aquatic environments. Therefore, it is very important to study the amount of these harmful elements and metals in fish that directly affect human health.
Totally, 405 fish from nine species with different feeding behaviors were dissected and their brain tissue was tested for the presence of 36 elements including antimony (Sb), arsenic (As), silver (Ag), aluminum (Al), bismuth (Bi), Barium (Ba), beryllium (Be), cesium (Ce), cobalt (Co),calcium (Ca),copper (Cu), iron (Fe), chromium (Cr), cadmium (Cd), lithium (Li), potassium (K), manganese (Mn), thorium (Th), magnesium (Mg), vanadium (V), lanthanum (La), rubidium (Rb), phosphorus (P), nickel (Ni), molybdenum (Mo), uranium (U), sodium (Na), lead (Pb), scandium (Sc), yttrium (Y), silicon (Si), sulfur (S), strontium (Sr), tin (Sn), titanium (Ti), tungsten (W), and zinc (Zn).
Calcium (Ca) exhibited different levels in brain tissues of the carnivorous, molluscivorous, piscivorous, and zooplanktivorous fish species (P < 0.05; Table 1), displaying distinct amounts between molluscivores and zooplanktivores as well as between zooplanktivores and carnivores (Table 2). The variation of TE accumulations in the brain of the above-mentioned dietary fish groups by PCA was decreased in two components (PC1 = 63.59%, PC2 = 17.68%) (Figs. 2 and Figs. 3), which determined that 81.27% of the total variability was correlated with TEs such as K, Mg, P, S, Zn, and Al. The two-dimensional diagrams showed the weight of any component in PCA which was mostly affected by Mg, K, P, and Zn, in PC1 while the highest value belonged to Al in PC2 (Figs. 2 and Figs. 3, Table 3).
Table 1.
Concentration of Trace Elements in the Brain of Different Feeding Types of Fish in the Southwest of the Caspian Basin
Elemental
Variables (ppm)
|
Mean ± SE
|
|
Piscivores
|
Molluscivores
|
Carnivores
|
Zooplanktivores
|
P
Value
|
| Al |
0.05 ± 0.05 |
0.19 ± 0.05 |
0.05 ± 0.03 |
0.13 ± 0.13 |
0.31 |
| Ca |
11.18 ± 5.08 |
220.95 ± 198.54 |
92.60 ± 68.11 |
9.86 ± 2.33 |
0.04*
|
| Cr |
0.00 ± 0.00 |
0.05 ± 0.03 |
0.05 ± 0.01 |
0.01 ± 0.01 |
0.08 |
| Fe |
0.41 ± 0.08 |
1.86 ± 1.15 |
0.61 ± 0.20 |
0.34 ± 0.18 |
0.18 |
| K |
34.70 ± 9.30 |
72.27 ± 31.36 |
62.76 ± 15.92 |
18.28 ± 7.96 |
0.22 |
| Mg |
3.35 ± 0.65 |
10.67 ± 4.36 |
8.46 ± 1.84 |
3.38 ± 0.99 |
0.13 |
| Na |
23.75 ± 12.25 |
28.87 ± 9.43 |
80.00 ± 23.74 |
21.15 ± 9.59 |
0.23 |
| P |
45.00 ± 24.90 |
110.80 ± 55.63 |
120.92 ± 44.21 |
25.73 ± 11.34 |
0.26 |
| S |
24.25 ± 5.25 |
59.43 ± 25.54 |
51.02 ± 13.59 |
14.35 ± 6.63 |
0.25 |
| Si |
0.00 ± 0.00 |
0.21 ± 0.05 |
0.15 ± 0.04 |
0.09 ± 0.06 |
0.12 |
| Sr |
0.10 ± 0.10 |
1.92 ± 1.71 |
0.49 ± 0.28 |
0.09 ± 0.03 |
0.13 |
| Zn |
0.55 ± 0.05 |
1.17 ± 0.41 |
0.84 ± 0.22 |
0.40 ± 0.07 |
0.19 |
*P < 0.05 was considered statistically significant.
Table 2.
Pai-wise Comparison (Mann–Whitney U-test) of the Ca Level Among the Four Feeding Types of Fish
|
Feeding type
|
M-W
|
Z
|
P
|
| Piscivores-Molluscivores |
0.00 |
-1.73 |
0.08 |
| Piscivores-Carnivoroes |
1.00 |
-1.39 |
0.17 |
| Piscivores-Zooplanktivores |
3.00 |
-0.46 |
0.64 |
| Molluscivores-Carnivores |
4.00 |
-0.71 |
0.48 |
| Molluscivores-Zooplanktivores |
0.00 |
-2.12 |
<0.05 |
| Carnivores-Zooplanktivores |
0.00 |
-2.31 |
<0.05 |
M-W and Z are grouping variable scores for Mann-Whitney and Kruskal-Wallis tests. P < 0.05 was considered statistically significant.
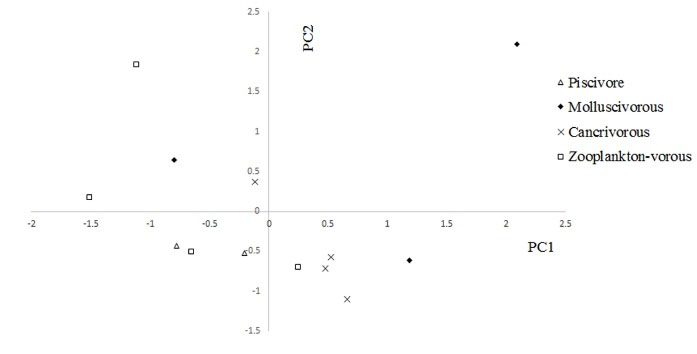
Figure 2.
Principal Component Analysis (PCA) of Trace Elements (TE) Concentrations in the Brain of Different Feeding Types of Fish from the Southwest the Caspian Basin. The scatter plots show fish scores for PC1 vs. PC2, which together describe the total variance of 81.27%.
.
Principal Component Analysis (PCA) of Trace Elements (TE) Concentrations in the Brain of Different Feeding Types of Fish from the Southwest the Caspian Basin. The scatter plots show fish scores for PC1 vs. PC2, which together describe the total variance of 81.27%.
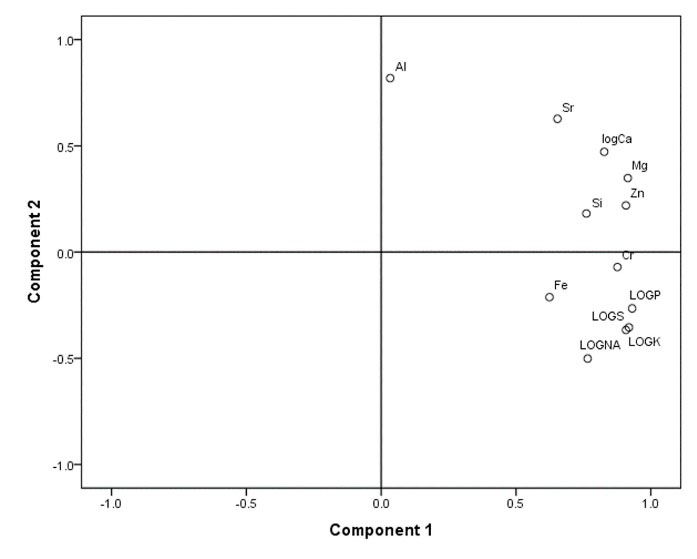
Figure 3.
Traits Loadings for PC1 and PC2 Resulting from Principal Components Analysis (PCA) of Multi-trace Elemental Investigation of the Brain of Different Feeding Types of Fish from the Southwest of Caspian Basin.
.
Traits Loadings for PC1 and PC2 Resulting from Principal Components Analysis (PCA) of Multi-trace Elemental Investigation of the Brain of Different Feeding Types of Fish from the Southwest of Caspian Basin.
Table 3.
Characteristic Loadings for PC1 and PC2 Resulting from PCA for Trace Elements Concentrations of the Brain in Different Feeding Types of Fish in the Southwest of the Caspian Basin
Elemental
Variables
|
PC1
|
PC2
|
| Al |
0.033 |
0.819 |
| Ca |
0.827 |
0.472 |
| Cr |
0.876 |
-0.070 |
| Fe |
0.624 |
-0.212 |
| K |
0.908 |
-0.367 |
| Mg |
0.915 |
0.349 |
| Na |
0.766 |
-0.502 |
| P |
0.931 |
-0.265 |
| S |
0.919 |
-0.355 |
| Si |
0.761 |
0.181 |
| Sr |
0.654 |
0.628 |
| Zn |
0.908 |
0.219 |
The matrix composed of element concentrations in brain tissue of fish species with different food items was described with two discriminant components (Wilk’s Lambda = 0.06, χ2 = 25.49, df = 6, and P < 0.05) (Fig. 4). Cross-validated grouping in DFA was 77% more successful than simple replacement law. The dendrogram (Fig. 5) discriminated molluscivores from other feeding types. Although zooplanktivores were observed in two sub-groups, carnivores were assigned only to one group which was distinct from piscivores and zooplanktivores.
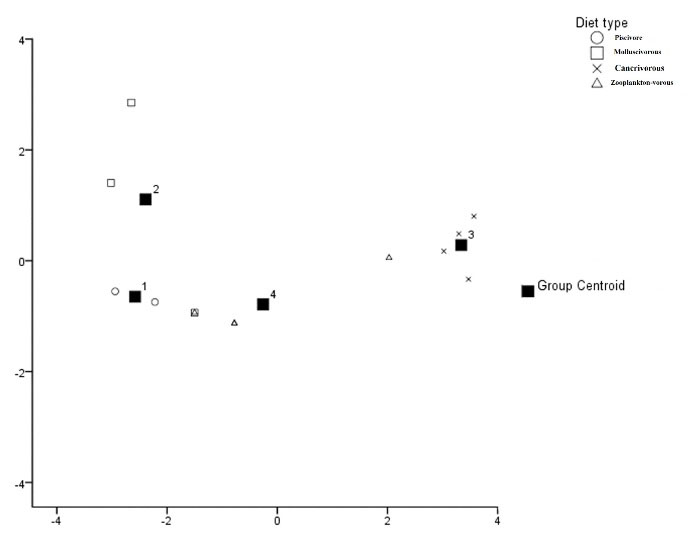
Figure 4.
Scheme of Discriminant Operation 1 and 2 for the Trace Elements Concentrations of the Brain of Different Feeding Types of Fish from the Inshore Water of Caspian Basin.
.
Scheme of Discriminant Operation 1 and 2 for the Trace Elements Concentrations of the Brain of Different Feeding Types of Fish from the Inshore Water of Caspian Basin.
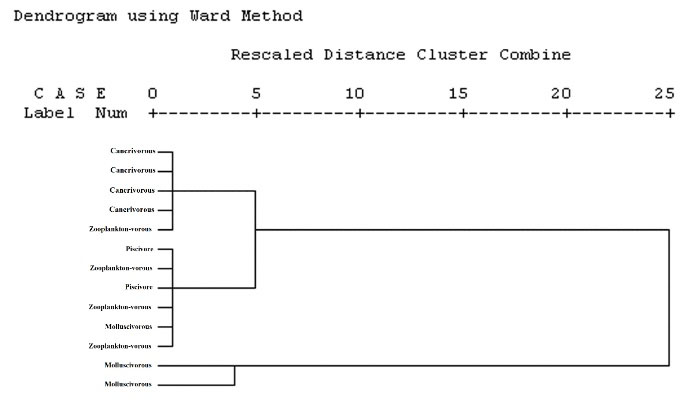
Figure 5.
Dendrogram Derived from Cluster Analysis of Trace Elements Concentrations of the Brain of Different Feeding Types of Fish from the Southwest of the Caspian Basin.
.
Dendrogram Derived from Cluster Analysis of Trace Elements Concentrations of the Brain of Different Feeding Types of Fish from the Southwest of the Caspian Basin.
In this study, we evaluated TEs accumulation in the fish brain as an indicator for different fish food items. Habitat, biological, physiological, and environmental parameters seem to be the reasons for different accumulation levels of TEs in different types of fish food items (30,31). Overall, some parameters such as food items, age, size, genetics, and habitat influence TE accumulation in different tissues such as the organism’s brain (32,33). Most TEs enter the body via consumption of food items, which is an important way of TE accumulation inside the tissues (34,35). The TE concentrations in these tissues depend on so many parameters such as the kind of feed, biological characteristics (such as metabolic rate, swimming behavior, species, etc.), adsorption and disposal chemical characteristics, kinetics, TE bioavailability, environmental circumstances (alkalinity, temperature, salinity, etc.) and TE concentration in sediments and water (36). The bioavailability of TEs due to feeding is mostly dependent upon the feed structure and considerably varied among different fish species (37).
In Scorpaena porcus, as a sessile species (38), measuring TEs concentrations and isotopic compounds in its muscle reflects the food items consumed. Predatory fish in comparison with non-predatory fish, tend to accumulate mercury, while benthic fish tend to accumulate cadmium (39,40). Briefly, there is a significant relationship between the food items of the aquatic organisms and the TEs concentration in their bodies (39). The local water contamination with some heavy metals such as mercury (Hg), in turn, leads to an increasing level of some elements in organisms inhabiting these localities (41) because the degree of contamination with the mercury in the sediments of the Caspian Sea was high; therefore, as expected, the concentration of this metal in carps was also high (30).
With the increase of size and age of the aquatic organism, a considerable increase in nitrogen (N) concentration was found, albeit with no detectable carbon (C) elevation. The elevated feeding levels in larger and older aquatic organisms are related to alterations in their food items and increased consumed dietary N (42).
Perga and Gray (43) observed different growth rates between adult and young fish based on morphological and physiological characters. The larger size of the fish mouth allows fish to ingest a wider range of preys with larger sizes such as Brachyura, Caridea, and Teleostei (44-47) to afford their requirements (38), which in turn influence the accumulation of TEs in their body.
In the present study, significant differences in the level of most TEs were found based on fish food items and species. In the literature, gender exhibited no influence on TE accumulation, so we did not consider it. According to previous studies, As and Hg levels were higher in adult fish than in younger ones, while Cd, Ba, Pb, and Cu levels were reported to be higher in younger fish than in adults. Fish age had no significant effect on Ni, Zn, and Cr levels (30). Significant correlations were found between fish age, size as well as total nitrogen and Hg concentration, exhibiting that TE bioaccumulation over their lifespan is related to their feeding level. It was not true for other elements (30). Our results were in line with those studies which indicated the positive correlation between nitrogen level and feeding rate (48-52).
The lack of correlations between some TEs and nitrogen level and also fish age exhibit that these elements did not accumulate via feed consumption during the organism’s life span, which is similar to results found by Ikemoto et al (54), Hao et al (55), and Zhang and Wang (56), while other studies revealed accumulation of As, Cd, and Pb in fresh and saltwater fish over their lifespan (57). However, some experimental studies displayed a low level of accumulation and a high level of dissipation of Cd and K, leading to a reduced potential of accumulation (58). However, their levels along with P, S, Zn, and Al exhibited marked alterations based on the fish food items. It was also true for Cd accumulation reported by other authors (59). Similar results were reported about the correlation of As and nitrogen in fishes of the North Atlantic Ocean (60,61).
Genetics besides food items is the other effective parameter influencing TEs accumulations in the organism tissues (62). Few reports are available about the effect of genetic characteristics on the TEs accumulation in humans and other animals, indicating that most of them were related to Cu and Zn. Ecotype influences TE composition in animal tissues. Therefore, their concentrations seem to be controlled by genetic characteristics (63,64). In the tissues of various animal species, the Cu, Mn, F, and Si levels were reported to be different from each other (65).
As mentioned before, gender is one of the useful parameters influencing TE accumulation in the organism tissues. Schepers (66) by injecting tetraethyllead and tetramethyl lead to rats found marked variations in the male and female rats in terms of Pb concentrations in their brain, liver, spleen, kidney, lung, and muscle. The Pb accumulations in all of these tissues (except for muscle) were higher in male rats than in females.
Soares et al (67) fed rats with different doses of methyl mercury (MeHg) and found that its accumulations in male and female rat were different, indicating that male had higher levels of Hg than female. The effects of gender on TE composition in the blood and bone of male and female chicken have been studied by Vo et al (68). They found that there are significant differences in terms of the Mg, Ca, Na, and Cl levels in the blood, as well as the concentrations of Ca, Na, Mg, Zn, Fe, Mn, and P in bones between males and females.
The physiological circumstances of aquatic organisms, especially hormones and pregnancy, are among the effective parameters in TE accumulation in their tissues. Many TEs cause alterations in element metabolism inside an organism’s tissues.
Injection of estrogen resulted in an increased Cu level and also elevated serum ceruloplasmin in many mammals (69,70). Furthermore, serum Cu levels increased in patients using contraceptive pills (71,72). Johnson et al (73) reported that testosterone leads to an increased serum Cu level in humans. Pregnancy also leads to elevated serum ceruloplasmin and consequently to an increased serum Cu level. Evans (74) suggested that elevated ceruloplasmin during pregnancy is, in fact, an effort to ensure the transmission of Fe and Cu to the growing fetus.
Diseases are also evident from TEs concentrations in the organism tissues. Different stressors lead to elevated TEs accumulations in many different species. However, exercise, inflammatory agents, and diseases are the most effective parameters (74).
Age is another parameter influencing TE accumulation in organism tissues. At present, neural damage due to aging is well known in monkeys and rodents. Many reports, for example, exhibited that TE composition is influenced by aging. Heumann and Leuba (75) reported that neuronal death (decrease in neurons) in the cerebral cortex of mouse occurs with age. It is also known that those mental diseases such as ischemia and its consequences may lead to elevated Ca and reduced K in the related tissue. Therefore, age-dependent alterations in K, Ca, and Rb levels may clearly lead to neural degeneration of the brain due to aging and the kind of food item. The organisms fed with food items belonged to low levels of the food chain than in piscivores which feed on the higher-level ones.
Habitat is also another factor affecting TEs accumulation in organisms. Feeding and also element concentrations are also notable parameters influencing TEs concentration in tissues of aquatic organisms. Little is known about the correlation between TEs accumulation in animal tissues and their concentrations in the ambient environment in the world. Environmental circumstances such as temperature are also effective factors in TEs concentrations inside the organism tissues. Additionally, these conditions exhibit their influences on calcium-containing tissues. Mueller (76) and de Andrade et al (77) reported that increased temperatures result in reduced Ca level in blood serum and consequent increase in Ca deposition inside some tissues. Therefore, at higher and more constant temperatures, aquatic organisms fed on mollusks or benthivores exhibit higher Ca level in their brain than those consumed zooplanktons and also pelagic preys.
4. Conclusion
In conclusion, while some TEs, including Zn, are necessary for brain function, some others such as Hg have adverse effects. The presence of TEs in different food item types reflects the importance of the TEs monitoring in environmental toxicology, health, and risk assessment. However, further studies on TEs accumulations in the fish brain are essential for the complete understanding of their role in fish brain activity as well as more surveys to understand long-term responses. Our study contributes towards these aims and sheds light on the specific roles of food item which need to be more investigated for the monitoring of aquatic contamination in fish besides genetic factors. Given the importance of human health, it is necessary to measure the amount of harmful metals that affect the health of the consumer. For this reason, it is better to do these studies annually. Accurate identification of contaminant source as well as determining the type of metal that enters the environment by contaminants can be of great help in such studies.
Authors’ Contributions
MS: Supervision, writing, review, and editing; MFV: Methodology, sample analysis, sampling, sample perpetration, investigation, writing; MB: Methodology, sample analysis, sampling, sample perpetration, investigation, writing; SB: Software, writing, review, and editing.
Conflict of Interest Disclosures
The authors declare that they have no conflict of interests.
Acknowledgments
The fund of this study with grant number 21195170 was provided by the Caspian Basin Research Center, University of Guilan, Rasht, Iran,
References
- Talukdar T, Zamroziewicz MK, Zwilling CE, Barbey AK. Nutrient biomarkers shape individual differences in functional brain connectivity: evidence from omega-3 PUFAs. Hum Brain Mapp 2019; 40(6):1887-97. doi: 10.1002/hbm.24498 [Crossref] [ Google Scholar]
- Zamroziewicz MK, Zwilling CE, Barbey AK. Inferior prefrontal cortex mediates the relationship between phosphatidylcholine and executive functions in healthy, older adults. Front Aging Neurosci 2016; 8:226. doi: 10.3389/fnagi.2016.00226 [Crossref] [ Google Scholar]
- Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci 2008; 9(7):568-78. doi: 10.1038/nrn2421 [Crossref] [ Google Scholar]
- Sandstead HH. Nutrition and brain function: trace elements. Nutr Rev 1987; 44:37-41. [ Google Scholar]
- Boran M, Karaçam H, Çelikkale MS, Köse S, Feyzioglu M, Kutlu S. Levels of heavy metals in blue whiting caught from the eastern Black Sea area of Turkey. Toxicol Environ Chem 2000; 75(1-2):67-73. doi: 10.1080/02772240009358893 [Crossref] [ Google Scholar]
- Pagano M, Porcino C, Briglia M, Fiorino E, Vazzana M, Silvestro S. The influence of exposure of cadmium chloride and zinc chloride on haemolymph and digestive gland cells from Mytilus galloprovincialis. Int J Environ Res 2017; 11(2):207-16. doi: 10.1007/s41742-017-0020-8 [Crossref] [ Google Scholar]
- Seco-Gesto EM, Moreda-Piñeiro A, Bermejo-Barrera A, Bermejo-Barrera P. Multi-element determination in raft mussels by fast microwave-assisted acid leaching and inductively coupled plasma-optical emission spectrometry. Talanta 2007; 72(3):1178-85. doi: 10.1016/j.talanta.2007.01.009 [Crossref] [ Google Scholar]
- Fazio F, Piccione G, Tribulato K, Ferrantelli V, Giangrosso G, Arfuso F. Bioaccumulation of heavy metals in blood and tissue of striped mullet in two Italian lakes. J Aquat Anim Health 2014; 26(4):278-84. doi: 10.1080/08997659.2014.938872 [Crossref] [ Google Scholar]
- Forouhar Vajargah M, Hedayati A. Acute toxicity of butachlor to Rutilus rutilus caspicus and Sander lucioperca in vivo condition. Transylv Rev Syst Ecol Res 2017; 19(3):85-92. doi: 10.1515/trser-2017-0023 [Crossref] [ Google Scholar]
- Forouhar Vajargah M, Mohamadi Yalsuyi A, Sattari M, Hedayati A. Acute toxicity effect of glyphosate on survival rate of common carp, Cyprinus carpio. Environ Health Eng Manag 2018; 5(2):61-6. doi: 10.15171/ehem.2018.09 [Crossref] [ Google Scholar]
- Forouhar Vajargah M, Mohammadi Yalsuyi A, Hedayati A. Acute toxicity of povidone-iodine (Betadine) in common carp (Cyprinus carpio L 1758). Pollution 2017; 3(4):589-93. doi: 10.22059/poll.2017.62775 [Crossref] [ Google Scholar]
- Muñoz-Olivas R, Cámara C. Speciation related to human health. In: Ebdon L, Pitts L, Cornelis R, Crews H, Donad OFX, Quevauviller P, eds. Trace Element Speciation for Environment, Food and Health. The Royal Society of Chemistry; 2001. p. 331-53.
- Vajargah MF, Hedayati A. Toxicity effects of cadmium in grass carp (Ctenopharyngodon idella) and Big Head Carp (Hypophthalmichthys nobilis). Transylvanian Review of Systematical and Ecological Research 2017; 19(1):43-48. doi: 10.1515/trser-2017-0004 [Crossref] [ Google Scholar]
- de Mora S, Sheikholeslami MR, Wyse E, Azemard S, Cassi R. An assessment of metal contamination in coastal sediments of the Caspian Sea. Mar Pollut Bull 2004; 48(1-2):61-77. doi: 10.1016/s0025-326x(03)00285-6 [Crossref] [ Google Scholar]
- Sadeghirad M. Accumulation of Trace Elements in Acipenser persicus Tissues in Relation to Feeding Habits and Mode of Absorption of These Pollutants. Iranian Fisheries Research Organisation, Internatinoal Sturgeon Research Institute; 2007. p. 42.
- Amini Ranjbar G, Sotudehnia F. Investigation of heavy metals accumulation in muscle tissue of Mugil auratus in relation to standard length, weight, age and sex. Iran J Fish Sci 2005; 14(3):1-18. [ Google Scholar]
- Fazeli MS, Abtahi B, Sabbagh kashani A. Assessing Pb, Ni and Zn accumulation in the tissues of Liza aurata in the south Caspian Sea. Iran J Fish Sci 2005; 14(1):65-78. [ Google Scholar]
- Ebrahimzadeh MA, Eslami S, Nabavi SF, Nabavi SM. Determination of trace element level in different tissues of the leaping mullet (Liza saliens, Mugilidae) collected from Caspian Sea. Biol Trace Elem Res 2011; 144(1-3):804-11. doi: 10.1007/s12011-011-9095-9 [Crossref] [ Google Scholar]
- Beheshti M. Comparative Study of Concentration of Heavy Metals (Cu, Fe, Zn, Mn) in Muscle, Liver and Gill Organ of Fish (Liza abu) in the Karoon and Karkheh Rivers in Khuzestan Province [thesis]. Ahwaz, Iran: Islamic Azad University, Science and Research Branch; 2011.
- Askary Sary A, Beheshti M. Cadmium, iron, lead and mercury bioaccumulation in abu mullet, Liza abu, different tissues from Karoun and Karkheh rivers, Khozestan, Iran. Bull Environ Contam Toxicol 2012; 88(2):158-61. doi: 10.1007/s00128-011-0476-8 [Crossref] [ Google Scholar]
- Alipour H, Banagar G. Health risk assessment of selected heavy metals in some edible fishes from Gorgan Bay, Iran. Iran J Fish Sci 2018; 17(1):21-34. doi: 10.22092/ijfs.2018.115582 [Crossref] [ Google Scholar]
- Khanipour AA, Ahmadi M, Seifzadeh M. Study on bioaccumulation of heavy metals (cadmium, nickel, zinc and lead) in the muscle of wels catfish (Silurus glanis) in the Anzali wetland. Iran J Fish Sci 2018; 17(1):244-50. doi: 10.22092/ijf.s.2018.118782 [Crossref] [ Google Scholar]
- Eslami S, Hajizadeh Moghaddam A, Jafari N, Nabavi SF, Nabavi SM, Ebrahimzadeh MA. Trace element level in different tissues of Rutilus frisii kutum collected from Tajan River, Iran. Biol Trace Elem Res 2011; 143(2):965-73. doi: 10.1007/s12011-010-8885-9 [Crossref] [ Google Scholar]
- Mirzajani AR, Hamidian AH, Karami M. Metal bioaccumulation in representative organisms from different trophic levels of the Caspian Sea. Iran J Fish Sci 2016; 15(3):1027-43. [ Google Scholar]
- Zar JH. Biostatistical Analysis. 3rd ed. New Jersey: Prentice Hall; 1996.
- Quinn GP, Keough MJ. Experimental Design and Data Analysis for Biologists. Cambridge, UK: Cambridge University Press; 2002.
- Bibak M, Sattari M, Agharokh A, Tahmasebi S, Imanpour Namin J. Assessing some heavy metals pollutions in sediments of the northern Persian Gulf (Bushehr province). Environ Health Eng Manag 2018; 5(3):175-9. doi: 10.15171/ehem.2018.24 [Crossref] [ Google Scholar]
- Bibak M, Sattari M, Tahmasebi S, Kafaei R, Sorial GA, Ramavandi B. Trace and major elements concentration in fish and associated sediment-seawater, northern shores of the Persian Gulf. Biol Trace Elem Res. 2020. 10.1007/s12011-020-02370-x.
- Sattari M, Bibak M, Bakhshalizadeh S, Forouhar Vajargah M. Element accumulations in liver and kidney tissues of some bony fish species in the southwest Caspian Sea. J Cell Mol Res 2020; 12(1):33-40. doi: 10.22067/jcmr.v12i1.85975 [Crossref] [ Google Scholar]
- Sattari M, Bibak M, Forouhar Vajargah M. Evaluation of trace elements contaminations in muscles of Rutilus kutum (Pisces: Cyprinidae) from the Southern shores of the Caspian Sea. Environ Health Eng Manag 2020; 7(2):89-96. doi: 10.34172/ehem.2020.11 [Crossref] [ Google Scholar]
- Evans MS, Lockhart WL, Doetzel L, Low G, Muir D, Kidd K. Elevated mercury concentrations in fish in lakes in the Mackenzie River Basin: the role of physical, chemical, and biological factors. Sci Total Environ 2005; 351-352:479-500. doi: 10.1016/j.scitotenv.2004.12.086 [Crossref] [ Google Scholar]
- Nasrollahzadeh Saravi H, Pourgholam R, Pourang N, Rezaei M, Makhlough A, Unesipour H. Heavy metal concentrations in edible tissue of Cyprinus carpio and its target hazard quotients in the Southern Iranian Caspian Sea coast, (2010). Journal of Mazandaran University of Medical Sciences 2013; 23(103):33-44. [ Google Scholar]
- Shokrzadeh M, Saeedi Saravi SS. The study of heavy metals (lead, cadmium, and chromium) in three species of most consumed fish sampled from Gorgan coast (Iran), 2008. Toxicol Environ Chem 2010; 92(1):71-3. doi: 10.1080/02772240902830730 [Crossref] [ Google Scholar]
- Pazooki J, Ghaffar HF, Abtahi B. A comparison of heavy metal concentrations in skin and muscle tissues of wild and cultured carp (Cyprinus carpio) in the southeastern Caspian Sea area of Iran. Environ Sci 2011; 9(1):51-8. [ Google Scholar]
- Bibak M, Tahmasebi S, Sattari M, Kafaei R, Ramavandi B. Empirical cumulative entropy as a new trace elements indicator to determine the relationship between algae-sediment pollution in the Persian Gulf, southern Iran. Environ Sci Pollut Res Int. 2020. 10.1007/s11356-020-10838-5.
- Mashroofeh A, Riyahi Bakhtiari A, Pourkazemi M. Evaluation of cadmium, vanadium, nickel and zink concentrations in different tissues of beluga and stellate sturgeon and risk assessment regarding consuming their muscle tissue in south Caspian Sea. Journal of Mazandaran University of Medical Sciences 2013; 22(96):89-96. [ Google Scholar]
- Willis JN, Sunda WG. Relative contributions of food and water in the accumulation of zinc by two species of marine fish. Mar Biol 1984; 80(3):273-9. doi: 10.1007/bf00392822 [Crossref] [ Google Scholar]
- Hall BD, Bodaly RA, Fudge RJP, Rudd JWM, Rosenberg DM. Food as the dominant pathway of methylmercury uptake by fish. Water Air Soil Pollut 1997; 100(1):13-24. doi: 10.1023/a:1018071406537 [Crossref] [ Google Scholar]
- Xu Y, Wang WX. Exposure and potential food chain transfer factor of Cd, Se and Zn in marine fish Lutjanus argentimaculatus. Mar Ecol Prog Ser 2002; 238:173-86. doi: 10.3354/meps238173 [Crossref] [ Google Scholar]
- Canli M, Atli G. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ Pollut 2003; 121(1):129-36. doi: 10.1016/s0269-7491(02)00194-x [Crossref] [ Google Scholar]
- Luoma SN, Hogstrand C, Bell RA, Bielmyer GK, Galvez F, LeBlanc GA, et al. Biological Processes. In: Andren AW, Bober TW, eds. Silver in the Environment: Transport, Fate, and Effects. Pensacola, FL: SETAC; 2002. p. 65-95.
- Bell JD, Harmelin-Vivien ML. Fish fauna of French Mediterranean Posidonia oceanica seagrass meadows II: feeding habits. Tethys (Marseille) 1983; 11(1):1-14. [ Google Scholar]
- Kidwell JM, Phillips LJ, Birchard GF. Comparative analyses of contaminant levels in bottom feeding and predatory fish using the National Contaminant Biomonitoring Program data. Bull Environ Contam Toxicol 1995; 54(6):919-23. doi: 10.1007/bf00197979 [Crossref] [ Google Scholar]
- Voigt HR. Concentrations of Mercury (Hg) and Cadmium (Cd), and the Condition of Some Coastal Baltic Fishes [dissertation]. Helsinki: Environmental Protection Science, University of Helsinki; 2004.
- Jezierska B, Witeska M. Metal toxicity to fish. In: Monografie. University of Podlasie (Poland); 2001.
- Harmelin-Vivien ML, Kaim-Malka RA, Ledoyer M, Jacob-Abraham SS. Food partitioning among scorpaenid fishes in Mediterranean seagrass beds. J Fish Biol 1989; 34(5):715-34. doi: 10.1111/j.1095-8649.1989.tb03352.x [Crossref] [ Google Scholar]
- Sattari M, Imanpour Namin J, Bibak M, Forouhar Vajargah M, Bakhshalizadeh S, Faggio C. Determination of Trace Element Accumulation in Gonads of Rutilus kutum (Kamensky, 1901) from the South Caspian Sea Trace Element Contaminations in Gonads. Proc Natl Acad Sci India Sect B Biol Sci 2020; 90(4):777-84. doi: 10.1007/s40011-019-01150-5 [Crossref] [ Google Scholar]
- Perga ME, Grey J. Laboratory measures of isotope discrimination factors: comments on Caut, Angulo & Courchamp (2008, 2009). J Appl Ecol 2010; 47(4):942-7. doi: 10.1111/j.1365-2664.2009.01730.x [Crossref] [ Google Scholar]
- Stergiou KI, Karpouzi VS. Feeding habits and trophic levels of Mediterranean fish. Rev Fish Biol Fish 2002; 11(3):217-54. doi: 10.1023/a:1020556722822 [Crossref] [ Google Scholar]
- Chouvelon T, Caurant F, Cherel Y, Simon-Bouhet B, Spitz J, Bustamante P. Species-and size-related patterns in stable isotopes and mercury concentrations in fish help refine marine ecosystem indicators and provide evidence for distinct management units for hake in the Northeast Atlantic. ICES J Mar Sci 2014; 71(5):1073-87. doi: 10.1093/icesjms/fst199 [Crossref] [ Google Scholar]
- Bibak M, Sattari M, Agharokh A, Tahmasebi S, Imanpour Namin J. Marine macro algae as a bio-indicators of heavy metal pollution in the marine environments, Persian Gulf. Indian J Mar Sci 2020; 49(3):357-63. [ Google Scholar]
- Cresson P, Fabri MC, Bouchoucha M, Brach Papa C, Chavanon F, Jadaud A. Mercury in organisms from the Northwestern Mediterranean slope: importance of food sources. Sci Total Environ 2014; 497-498:229-38. doi: 10.1016/j.scitotenv.2014.07.069 [Crossref] [ Google Scholar]
- Campbell LM, Norstrom RJ, Hobson KA, Muir DC, Backus S, Fisk AT. Mercury and other trace elements in a pelagic Arctic marine food web (Northwater Polynya, Baffin Bay). Sci Total Environ 2005; 351-352:247-63. doi: 10.1016/j.scitotenv.2005.02.043 [Crossref] [ Google Scholar]
- Ikemoto T, Tu NP, Okuda N, Iwata A, Omori K, Tanabe S. Biomagnification of trace elements in the aquatic food web in the Mekong Delta, South Vietnam using stable carbon and nitrogen isotope analysis. Arch Environ Contam Toxicol 2008; 54(3):504-15. doi: 10.1007/s00244-007-9058-5 [Crossref] [ Google Scholar]
- Hao Y, Chen L, Zhang X, Zhang D, Zhang X, Yu Y. Trace elements in fish from Taihu Lake, China: levels, associated risks, and trophic transfer. Ecotoxicol Environ Saf 2013; 90:89-97. doi: 10.1016/j.ecoenv.2012.12.012 [Crossref] [ Google Scholar]
- Zhang W, Wang WX. Large-scale spatial and interspecies differences in trace elements and stable isotopes in marine wild fish from Chinese waters. J Hazard Mater 2012; 215-216:65-74. doi: 10.1016/j.jhazmat.2012.02.032 [Crossref] [ Google Scholar]
- Dierking J, Wafo E, Schembri T, Lagadec V, Nicolas C, Letourneur Y. Spatial patterns in PCBs, pesticides, mercury and cadmium in the common sole in the NW Mediterranean Sea, and a novel use of contaminants as biomarkers. Mar Pollut Bull 2009; 58(11):1605-14. doi: 10.1016/j.marpolbul.2009.07.008 [Crossref] [ Google Scholar]
- Nfon E, Cousins IT, Järvinen O, Mukherjee AB, Verta M, Broman D. Trophodynamics of mercury and other trace elements in a pelagic food chain from the Baltic Sea. Sci Total Environ 2009; 407(24):6267-74. doi: 10.1016/j.scitotenv.2009.08.032 [Crossref] [ Google Scholar]
- Cossa D, Harmelin-Vivien M, Mellon-Duval C, Loizeau V, Averty B, Crochet S. Influences of bioavailability, trophic position, and growth on methylmercury in hakes (Merluccius merluccius) from Northwestern Mediterranean and Northeastern Atlantic. Environ Sci Technol 2012; 46(9):4885-93. doi: 10.1021/es204269w [Crossref] [ Google Scholar]
- Asante KA, Agusa T, Mochizuki H, Ramu K, Inoue S, Kubodera T. Trace elements and stable isotopes (delta13C and delta15N) in shallow and deep-water organisms from the East China Sea. Environ Pollut 2008; 156(3):862-73. doi: 10.1016/j.envpol.2008.05.020 [Crossref] [ Google Scholar]
- Sattari M, Imanpour Namin J, Bibak M, Forouhar Vajargah M, Khosravi A. Investigation of metal element concentrations in tissue of Rutilus frisii in the Southwest Caspian Sea. Iran J Fish Sci 2019; 28(3):149-61. doi: 10.22092/isfj.2019.119162 [Crossref] [ Google Scholar]
- Wang WX. Interactions of trace metals and different marine food chains. Mar Ecol Prog Ser 2002; 243:295-309. doi: 10.3354/meps243295 [Crossref] [ Google Scholar]
- Dietz R, Riget F, Cleemann M, Aarkrog A, Johansen P, Hansen JC. Comparison of contaminants from different trophic levels and ecosystems. Sci Total Environ 2000; 245(1-3):221-31. doi: 10.1016/s0048-9697(99)00447-7 [Crossref] [ Google Scholar]
- Asante KA, Agusa T, Kubota R, Mochizuki H, Ramu K, Nishida S. Trace elements and stable isotope ratios (delta(13)C and delta(15)N) in fish from deep-waters of the Sulu Sea and the Celebes Sea. Mar Pollut Bull 2010; 60(9):1560-70. doi: 10.1016/j.marpolbul.2010.04.011 [Crossref] [ Google Scholar]
- Lüchmann KH, Dafre AL, Trevisan R, Craft JA, Meng X, Mattos JJ. A light in the darkness: new biotransformation genes, antioxidant parameters and tissue-specific responses in oysters exposed to phenanthrene. Aquat Toxicol 2014; 152:324-34. doi: 10.1016/j.aquatox.2014.04.021 [Crossref] [ Google Scholar]
- Lucis OJ, Lucis R. Distribution of cadmium 109 and zinc 65 in mice of inbred strains. Arch Environ Health 1969; 19(3):334-6. doi: 10.1080/00039896.1969.10666853 [Crossref] [ Google Scholar]
- Soares JH Jr, Miller D, Lagally H, Stillings BR, Bauersfeld P, Cuppett S. The comparative effect of oral ingestion of methyl mercury on chicks and rats. Poult Sci 1973; 52(2):452-8. doi: 10.3382/ps.0520452 [Crossref] [ Google Scholar]
- Vo KV, Boone MA, Torrence AK. Electrolyte content of blood and bone in chickens subjected to heat stress. Poult Sci 1978; 57(2):542-4. doi: 10.3382/ps.0570542 [Crossref] [ Google Scholar]
- March BE, Soong R, Bilinski E, Jonas RE. Effects on chickens of chronic exposure to mercury at low levels through dietary fish meal. Poult Sci 1974; 53(6):2175-81. doi: 10.3382/ps.0532175 [Crossref] [ Google Scholar]
- Underwood EJ. Trace Elements in Human and Animal Nutrition. 4th ed. New York: Academic Press; 1977.
- Schepers GW. Tetraethyllead and tetramethyllead Comparative experimental pathology: I Lead absorption and pathology. Arch Environ Health 1964; 8:277-95. doi: 10.1080/00039896.1964.10663668 [Crossref] [ Google Scholar]
- Johnson NC, Kheim T, Kountz WB. Influence of sex hormones on total serum copper. Proc Soc Exp Biol Med 1959; 102:98-9. doi: 10.3181/00379727-102-25155 [Crossref] [ Google Scholar]
- German JL 3rd, Bearn AG. Effect of estrogens on copper metabolism in Wilson’s disease. J Clin Invest 1961; 40(3):445-53. doi: 10.1172/jci104272 [Crossref] [ Google Scholar]
- Evans GW. Copper homeostasis in the mammalian system. Physiol Rev 1973; 53(3):535-70. doi: 10.1152/physrev.1973.53.3.535 [Crossref] [ Google Scholar]
- Heumann D, Leuba G. Neuronal death in the development and aging of the cerebral cortex of the mouse. Neuropathol Appl Neurobiol 1983; 9(4):297-311. doi: 10.1111/j.1365-2990.1983.tb00116.x [Crossref] [ Google Scholar]
- Mueller WJ. The effect of environmental temperature and humidity on the calcium balance and serum calcium of laying pullets. Poult Sci 1959; 38(6):1296-301. doi: 10.3382/ps.0381296 [Crossref] [ Google Scholar]
- de Andrade AN, Rogler JC, Featherston WR. Influence of constant elevated temperature and diet on egg production and shell quality. Poult Sci 1976; 55(2):685-93. doi: 10.3382/ps.0550685 [Crossref] [ Google Scholar]
- Forouhar Vajargah M, Sattari M, Imanpour Namin J, Bibak M. Length-weight, length-length relationships and condition factor of Rutilus kutum (Actinopterygii: Cyprinidae) from the southern Caspian Sea, Iran. J Animal Divers 2020; 2(2):56-61. doi: 10.29252/jad.2020.2.2.6 [Crossref] [ Google Scholar]