Avicenna J Environ Health Eng. 7(1):29-34.
doi: 10.34172/ajehe.2020.05
Original Article
Genetic Variation in Response to Global Warming in a Coral Reef Species, Porites lobata
Pegah Javid 1  , Naser Farrokhi 2, Siamak Behzadi 3, Mohammadreza Bakhtiarizadeh 4, Seyed Mehdi Alavi 5, Mohammad Sharif Ranjbar 1, *
, Naser Farrokhi 2, Siamak Behzadi 3, Mohammadreza Bakhtiarizadeh 4, Seyed Mehdi Alavi 5, Mohammad Sharif Ranjbar 1, * 
Author information:
1Department of Marine Biology, Faculty of Marine Science and Technology, University of Hormozgan, Bandar Abbas, Iran.
2Department of Cell and Molecular Biology, Faculty of Life Sciences and Biotechnology, Shahid Beheshti University, Tehran, Iran.
3Persian Gulf and Oman Sea Ecological Research Institute, Agricultural Research, Education and Extension Organization, Bandar Abbas, Iran.
4Department of Animal and Poultry Science, College of Aburaihan, University of Tehran, Tehran, Iran.
5Department of Plant Biotechnology, National Institute of Genetic Engineering and Biotechnology, Tehran, Iran.
Abstract
Climate change due to global warming is one of the worst environmental disasters in the world, which affects all ecosystems and has led to increasing degradation of coral reefs. The increase of sea surface temperature is inversely related to the resistance of corals and is directly associated with their bleaching. High temperature disrupts the symbiotic relationship between coral and algal symbiont and results in coral bleaching. To evaluate the adaptation of corals to heat stress, in this study, we investigated the thermal stress effect on the expression of genes involved in programmed cell death (PCD), cysteinyl aspartate proteases 3 (will be mentioned as Caspas3 hereafter) and anti-apoptotic pathway, B-cell lymphoma 2 (will be mentioned as Bcl2 hereafter) in Porites lobata (Dana, 1846). Corals were incubated at 25°C for 2 weeks (adaptation period) and then exposed to 34°C (heat shock) for 24 and 48 hours. Then, the expression of genes was measured using real-time PCR. The results revealed that both genes were up-regulated at 24 hours after heat induction. Bcl-2 expression (anti-apoptotic gene) was induced at 24 hours and was down-regulated at 48 hours. In contrast, Caspase3 (apoptotic gene) continued to be expressed up to 48 hours. These results might indicate that coral cells are headed towards bleaching and death with increased temperature. The results of this study, regarding the observed expression patterns, can clarify the response of different genes to a thermal stress in coral reefs. The exposure of corals to acute conditions with high temperatures presented the behavior of the desired genes in the studied conditions.
Keywords: Environmental deterioration, Apoptosis, Bcl-2, Caspase3, Real-time PCR, Thermal stress, Global change
Copyright and License Information
© 2020 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium provided the original work is properly cited.
1. Introduction
Stony corals are Cnidarians that have an obligated symbiotic relationship with algal photosynthetic symbionts of the genus Symbiodinium (1). Bleaching of coral reefs is closely associated with excessive thermal stress via disturbances in coral-algae symbiosis (2). It has been considered as the most serious threat to reefs in recent years (3). Bleaching of coral reefs adversely affects their health, growth, and reproduction and endangers their structure and function (4,5). Due to global warming and temperature increase, the frequency and scale of mass bleaching have increased over the last 40 years in different corals of the world (6) as well as the Persian Gulf (7) (Fig. 1).

Figure 1.
Coral Reefs of the Persian Gulf (Qeshm Island) after a Temperature Rise in Summer, Left: 2015, Right: 2017.
.
Coral Reefs of the Persian Gulf (Qeshm Island) after a Temperature Rise in Summer, Left: 2015, Right: 2017.
Since the environmental impact of global warming has been gradual, one expects to see adaptive responses for corals that can be mirrored at the physiological level (8). However, the thermal stress has had some irreversible effects on corals around the world through endangering both the hosts and the symbionts as they enter programmed cell death (PCD) (9). PCD, also known as apoptosis, in corals was first reported by Cikala et al (10). Apoptosis leads to widespread inflammation and genetic instability (11), increase in colony mortality and tissue necrosis, bleaching, discontinuity of skeletal growth, and decrease in larval survival, synthesis of proteins, and thickness of epithelial tissue (12). Evidence on some apoptotic genes suggests that PCD in corals is similar to that in vertebrates (13). The relevance between coral bleaching and the disease outbreak indicates that the inherent immune system of coral (as the host) is influenced by bleaching and long-term changes continue even after the end of tough conditions (14). Observations also indicate that apoptosis occurs simultaneously in symbionts and hosts under environmental stress (9).
Apoptosis is a mediated pathway that leads to the elimination of specific cells. This highly conserved process plays roles in biological and physiological functions of multicellular organisms. Its importance is obvious in developmental processes such as metamorphosis (15), symbiosis (16), tissue homeostasis, health, growth (17), response to stress, and defense against pathogens as well (18). Apoptosis is a complicated and pivotal biological process, which causes organisms to remove undesirable cells through disease or even homeostasis (19). Cikala et al (10) realized the apoptosis process in corals for the first time.
Bioinformatic evidence on some apoptotic genes suggests that coral apoptosis is similar to apoptosis in vertebrates (13). In apoptotic cascade, caspases, a family of cysteine proteases, are vital mediators of the inflammatory response and cell death through either formation of apoptotic bodies or cell destruction (20). In a counter-effective action, cells benefit from some anti-apoptotic genes, some of which act as oncogenes (21), which suppress PCD, mainly through the control of mitosis (22). Bcl-2 is an oncogene that has been proven to act as an apoptosis regulator (20,23,24). Considering that stresses influence a wide variety of biological, physiological, and molecular aspects of an organism, and immunity is the first system which organisms employ when they face a tension, in the current study, the effect of thermal stress on P. lobata was studied by the expression analyses of the representative of apoptotic (Caspase3) and anti-apoptotic (Bcl-2) genes in order to find out how high temperature can change the expression of genes involved in the immune system.
2. Materials and Methods
2.1 Sampling and Tissue Isolation
The samples of P. lobata were collected from the Persian Gulf, located in the southeast of Qeshm Island (26° 55.623’ N. 56° 15.377’ E), through SCUBA diving at a depth of 6 m. Small portions of P. lobata reef were prepared by graver and hammer. The live specimens were transferred to the simulation system at the Persian Gulf and Oman Sea Ecological Research Institute, Bandar Abbas, Iran in February 2019. Samples were adapted to laboratory conditions for two weeks at 25°C, a constant salinity of 35 psu, continuous water flow, and a 10 hour (light):14 hour (dark) photoperiod. The corals were fed once during the day with phytoplankton and once during the night with zooplankton (Deep Ocean, Iran). The tissue isolation from control specimens was carried out at 25°C in four biological replicates at the end of the 2-week adaptation period. The water temperature of treatment tanks increased to 34°C. It took almost 12 hours to increase the water temperature to 34°C. Tissue isolation from treated samples was performed at 24 and 48 hours after temperature adjustment in four replicates (totally 12 specimens). The tissues were put in cryovials containing RNAlater (Yekta Tajhiz, Tehran, Iran). They were kept at 4°C for 24 hours, snap-frozen in liquid nitrogen, and stored at -70°C.
2.2. RNA Isolation and cDNA Synthesis
RNA was isolated from tissues (100 mg) using Trizol (Ambion, Invitrogen Co., US) according to the manufacturer’s protocol. Quality and quantity of RNA samples were checked by 1.5% (w/v) agarose gel electrophoresis and NanoDrop spectrophotometer (Thermo Scientific, US), respectively. DNase Kit (SinaClone, Tehran, Iran) was used to remove DNA residuals. RNA (1 µg) was reverse transcribed into cDNA using cDNA synthesis kit (Yekta Tajhiz, Tehran, Iran) according to the manufacturer ‘s protocol.
2.3. Quantitative Polymerase Chain Reaction (qPCR)
Real-time PCR was carried out on cDNA samples in two technical replicates for each biological sample (totally 24 specimens) using gene specific primers (Table 1) in a 10 µL reaction volume. The reaction was conducted as follows: initial denaturation at 95°C for 10 minutes, followed by 45 cycles of [denaturation at 95°C for 30 seconds, annealing at 60°C for 30 seconds, and final extension at 72°C for 20 seconds]. Beta-actin (βAct) gene was used as the reference gene or internal control to normalize the data and Livak and Schmittgen (25) method was used to analyze the relative expression of genes.
Table 1.
Characteristics of the Primers Used in the Study
|
Primer
|
Forward
|
Reverse
|
Tm (°C)
|
Amplicon Size
|
|
β Act
|
5’- ATCATGAAGTGCGATGTGGA - 3’ |
5’- GGAGCAATGATCTTGATCTTCA - 3’ |
55.25
56.53 |
151 |
|
Caspase3
|
5’- TTATGATGCATTCATTTTCTCCA – 3’ |
5’ – GCTTGGAAGAAAAACATCTTTGG – 3’ |
53.52
57.08 |
150 |
|
Bcl-2
|
5’- ACACTTTTGCCGCAGTGG - 3’ |
5’ – TCGTTGAAGATAAAATCCACCA - 3’ |
55.97
54.66 |
171 |
The efficiency of the primers was determined using serial dilution test. Amplification efficiency (E) was determined for each target gene using the equation E(%) = (101/slope - 1) × 100, where the slope was estimated by plotting the Ct over the serial dilutions of cDNA. The expression of target genes relative to the reference genes was calculated by the formula 2–ΔΔCT to analyze the relative changes in gene expression. The prepared cDNA was serially diluted (1, 1/3, 1/9, 1/27 and 1/81).
The results of qRT-PCR showed relative mRNA expression level and normality was tested by Kolmogorov-Smirnov test. Differences in the expression of genes between different thermal treatments were analyzed by the one-way analysis of variance (ANOVA), followed by a Tukey’s multiple range test for multiple comparisons at the significant level of P<0.05. All statistical analyzes were performed using SPSS and SigmaPlot software packages.
3. Results and Discussion
Continuous exposure of the samples to heat shock for 48 hours resulted in bleaching of coral samples. It means that the color of coral samples faded after 48-hours of exposure to 34°C compared to the ones at the control temperature (25°C). The bleaching of samples indicates the disturbance in symbiotic relationship between algal symbionts and corals. However, the corals did not completely bleach until the end of the heat shock induction. This event was obvious in nature due to the climate change and thermal stress on coral reefs during the warm seasons. Therefore, global warming has led to bleaching and then death of many coral reefs in the Persian Gulf in 2017 (Fig. 1).
The results showed that gene expression levels were different in the whole experiment between Caspase3 and Bcl-2 although the expression of genes was not significant (P>0.05) compared to the reference gene. Fig. 2A and 2B indicates that the genes were both up-regulated at 24 hours after heat shock; however, the expression of Bcl-2 was at the maximum level and higher compared to Caspase3 at 24 hours. It seems that the gene expression increased from the initial time of the experiment to 24 hours after heat stress induction in both genes. Interestingly, the up-regulation of Caspase3 continued until 48 hours after heat stress induction (Fig. 2A), while the expression of Bcl-2 sharply decreased at 48 hours (Fig. 2B).
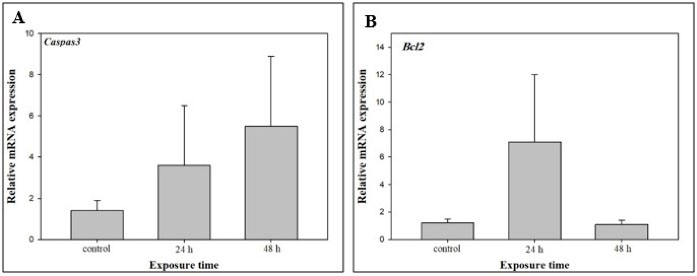
Figure 2.
Expression of the Apoptotic Gene Casp3 (A) and Anti-apoptotic Gene Bcl-2 (B) in a Coral Reef Species (Porites Lobata) in Response to Increased Temperature at 24 and 48 hours after Heat Shock Compared to Control Phase
.
Expression of the Apoptotic Gene Casp3 (A) and Anti-apoptotic Gene Bcl-2 (B) in a Coral Reef Species (Porites Lobata) in Response to Increased Temperature at 24 and 48 hours after Heat Shock Compared to Control Phase
Understanding the relationship between stress and immune system pathway can reveal the details of pathways involved in fighting a stress (26). Coral bleaching not only disrupts the symbiotic relationship between algae and coral but also affects different aspects of the physiology and ecology of corals (4,26,27). Studying coral responses to environmental changes through common tools such as transcriptomic and genomic techniques has revealed many facts about biological pathways. However, the environmental impacts on their immune system have remained vague (28,29). The levels of gene expression in bleached colonies provide evidence for some processes affecting corals (12). The immune system of corals has a vital role in resistance against stressors and this fact would improve predictions about the future status of corals (29,30,31). It is reported that apoptosis is active in various cellular processes in Hydra and bleaching in the anemone Aiptasia pallida (9,32). However, the evolutionary origin of these pathways is not clear yet.
Gene analysis has shown that pathways related to the immune system such as apoptosis are suppressed during and after bleaching (12). The relationship between coral bleaching and its immunity is complicated (14). Bleaching of corals affects the molecular system including the expression of different genes, especially inherent immune genes, and these reactions can continue up to one year after the event. This continuity of gene expression variations is shown at the whole transcriptome level, but the expression levels might be different (12). According to a study by Pinzón et al (12) on bleaching of a coral reef species (Orbicella faveolata) due to natural thermal stress, in a symbiotic relationship, the host and symbiont can have different responses to bleaching, and the host immune system might be suppressed even one year after a bleaching event. They showed that the casp8 expression was upregulated in bleached colonies of O. faveolata and it was downregulated after recovery of corals and obviously there were differences in expression of genes in bleached and unbleached colonies; less expression level of Casp8 gene was observed in white colonies as well (12). Studies on O. faveolata under thermal stress have shown that components of Casp8 pathway were suppressed in bleached colonies. Primary disruption of apoptosis in bleached colonies is likely to be due to bleaching control mechanisms. When the apoptosis is blocked, bleaching decreases (17,33). Although a mechanism for reduction of bleaching is conducted in corals, continuous downregulation of apoptosis could have an immunosuppressive effect almost one year after bleaching (12). Bleached colonies of O. faveolata have a higher prevalence of disease than unbleached colonies during and after bleaching (4). Environmental stressors suppress the immune system in other invertebrates as well (34). Depending on the intensity of apoptosis in the stressed specimen, its gene expression may vary in different tissues. In the sea anemone, for example, the expression of genes in endoderm was higher compared to ectoderm. This may be due to the specialized function of Caspase in different tissues. This gene is more expressed in endoderm-dependent processes even before symbiosis (13).
The difference in gene expression between unbleached and bleached colonies indicated that there must be dysfunction in the complement systems in bleached colonies that causes the immune system to become less active or inactive. The less active component system indicates the lack, reduction or suppression of immune system. The expression level of Casp8 has been lower in completely bleached corals (12).
Inducing the heat shock of 33°C to 34°C in a soft coral species (Aiptasia pallida) showed that activity of Caspas3 in tensioned samples increased by 45% compared to the control samples. It is noteworthy that Casp gene in A. pallida is homologous to the secondary structure of Caspase3 in vertebrates, and heat stress induces apoptosis in the sea anemone species (Aiptasiasp.) (9,17). It was shown that proteins of Bcl-2 family allow the assembly of apoptosome by modulating the release of cytochrome c from mitochondria while the apoptosome contains caspase-9 (35). Observations of inhibitory treatments indicate that Caspase activity can be inhibited by gene silencing (17). Most of Bcl-2 family domains are present in the apoptotic pathway (e.g., Bak and Bax) of vertebrates and they are clearly seen in sea anemones as soft corals and hard corals. Although many Bcl-2 proteins of corals appear to be orthologues of Bcl-2 specific proteins in mammals, the relationships of anti-apoptotic proteins in Acropora are unclear (35,36). In the current study, considering that Casp8 and Bcl-2 act in the opposite way in the apoptosis of cells, the expression trend of both was logically justifiable.
Studies have shown that the frequency of apoptotic cells in the host initially increases with enhancing thermal stress and then decreases. The reduction of apoptotic cells coincided with an increase in necrotic cells in corals. Examining more than 35 000 cells has shown that programmed death and necrosis would increase in response to heat stress over time (9). Thermal stress stimulates the pathways of cell death, which vary among different cells in extent and volume. The studies showed that the apoptosis rate increased in endodermal cells of A. pallida under heat shock of 33.5±0.5°C during the first 18 hours and decreased afterwards (9). This may be due to the high sensitivity of endodermal cells to environmental stresses. It means that the expression level of genes increased in the early stages of stress. The constant stress endangers the cell health and causes an increase in the expression level of caspase gene, thereby leading to cell death.
Since coral bleaching could be a drastic ecological event and a significant threat for coral reef ecosystems, understanding this process through adaptation and evolution of the cell death process is crucial (37). These observations show that the physiological tolerance of the holobiont as well as the reflection of different genetic compositions over time can define the overall condition of a colony (38,39).
This study indicated that the apoptotic pathway is present in Porites. Finding the apoptotic pathway of cnidarians can provide a better understanding of this pathway in higher vertebrates and mammals. Concurrent histological examinations in future studies may reveal a broader view of the mechanisms of apoptosis in corals as defense mechanisms.
4. Conclusion
In this study, coral reefs were used as indicators of environmental deterioration due to global warming. The expression of two genes associated with the apoptotic pathway (Casp3 as an apoptotic gene and Bcl-2 as an anti-apoptotic gene) in the coral reef species Porites lobata under the thermal stress was investigated at two distinct times (24 and 48 hours) using a high temperature of 34°C. The results of this study revealed that the expression patterns of these genes are consistent with the functions of these genes. The high expression level of gene Casp3 at 48 hours confirms the down-regulation of gene Bcl-2 at this time in response to stressful conditions in which leads to decreased resistance and death of P. lobata. The investigation of physiological pathways through molecular and genomic approaches could be the subject of future studies for interested researchers.
Conflict of Interest Disclosures
The authors declare that there is no conflict of interests.
Acknowledgment
This research could not have been carried out without the helps of Mr. Sajjad Pabasteh in sampling and preparing the simulation system and Dr. Arash Akbarzadeh for his consultation during the lab works and analyses. We are grateful to the Persian Gulf and Oman Sea Ecological Research Institute for providing a suitable place for holding the simulation system. We are also thankful to Mr. Behnam Vatankhah, Mr. Farshid Sharifian, and Mr. Hassan Jamshidi at the National Institute of Genetic Engineering and Biotechnology for their assistance in laboratory experiments and coordination”.
References
- Kenkel CD, Goodbody-Gringley G, Caillaud D, Davies SW, Bartels E, Matz MV. Evidence for a host role in thermotolerance divergence between populations of the mustard hill coral (Porites astreoides) from different reef environments. Mol Ecol 2013; 22(16):4335-48. doi: 10.1111/mec.12391 [Crossref] [ Google Scholar]
- Baird AH, Bhagooli R, Ralph PJ, Takahashi S. Coral bleaching: the role of the host. Trends Ecol Evol 2009; 24(1):16-20. doi: 10.1016/j.tree.2008.09.005 [Crossref] [ Google Scholar]
- Antonelli PL, Rutz SF, Sammarco PW, Strychar KB. A coral bleaching model. Nonlinear Anal Real World Appl 2014; 16:65-73. doi: 10.1016/j.nonrwa.2013.09.006 [Crossref] [ Google Scholar]
- Schnitzler CE, Hollingsworth LL, Krupp DA, Weis VM. Elevated temperature impairs onset of symbiosis and reduces survivorship in larvae of the Hawaiian coral, Fungia scutaria. Mar Biol 2012; 159(3):633-42. doi: 10.1007/s00227-011-1842-0 [Crossref] [ Google Scholar]
- Wooldridge SA. Breakdown of the coral-algae symbiosis: towards formalising a linkage between warm-water bleaching thresholds and the growth rate of the intracellular zooxanthellae. Biogeosciences 2013; 10(3):1647-58. doi: 10.5194/bg-10-1647-2013 [Crossref] [ Google Scholar]
- Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH. Global warming and recurrent mass bleaching of corals. Nature 2017; 543(7645):373-7. doi: 10.1038/nature21707 [Crossref] [ Google Scholar]
- Javid P, Soyouf Jahromi M, Ranjbar MS. The status of coral reefs in the Larak Island, Persian Gulf, from 2012 to 2018. Intern J Vet Anim Res 2018; 1(3):49-53. [ Google Scholar]
- Oliver TA, Palumbi SR. Do fluctuating temperature environments elevate coral thermal tolerance?. Coral Reefs 2011; 30(2):429-40. doi: 10.1007/s00338-011-0721-y [Crossref] [ Google Scholar]
- Dunn SR, Thomason JC, Le Tissier MD, Bythell JC. Heat stress induces different forms of cell death in sea anemones and their endosymbiotic algae depending on temperature and duration. Cell Death Differ 2004; 11(11):1213-22. doi: 10.1038/sj.cdd.4401484 [Crossref] [ Google Scholar]
- Cikala M, Wilm B, Hobmayer E, Böttger A, David CN. Identification of caspases and apoptosis in the simple metazoan Hydra. Curr Biol 1999; 9(17):959-62. doi: 10.1016/s0960-9822(99)80423-0 [Crossref] [ Google Scholar]
- Kültz D. Evolution of the cellular stress proteome: from monophyletic origin to ubiquitous function. J Exp Biol 2003; 206(Pt 18):3119-24. doi: 10.1242/jeb.00549 [Crossref] [ Google Scholar]
- Pinzón JH, Kamel B, Burge CA, Harvell CD, Medina M, Weil E. Whole transcriptome analysis reveals changes in expression of immune-related genes during and after bleaching in a reef-building coral. R Soc Open Sci 2015; 2(4):140214. doi: 10.1098/rsos.140214 [Crossref] [ Google Scholar]
- Dunn SR, Phillips WS, Spatafora JW, Green DR, Weis VM. Highly conserved caspase and Bcl-2 homologues from the sea anemone Aiptasia pallida: lower metazoans as models for the study of apoptosis evolution. J Mol Evol 2006; 63(1):95-107. doi: 10.1007/s00239-005-0236-7 [Crossref] [ Google Scholar]
- Mydlarz LD, Couch CS, Weil E, Smith G, Harvell CD. Immune defenses of healthy, bleached and diseased Montastraea faveolata during a natural bleaching event. Dis Aquat Organ 2009; 87(1-2):67-78. doi: 10.3354/dao02088 [Crossref] [ Google Scholar]
- Seipp S, Schmich J, Leitz T. Apoptosis--a death-inducing mechanism tightly linked with morphogenesis in Hydractina echinata (Cnidaria, Hydrozoa). Development 2001; 128(23):4891-8. [ Google Scholar]
- Dunn SR, Weis VM. Apoptosis as a post-phagocytic winnowing mechanism in a coral-dinoflagellate mutualism. Environ Microbiol 2009; 11(1):268-76. doi: 10.1111/j.1462-2920.2008.01774.x [Crossref] [ Google Scholar]
- Dunn SR, Schnitzler CE, Weis VM. Apoptosis and autophagy as mechanisms of dinoflagellate symbiont release during cnidarian bleaching: every which way you lose. Proc Biol Sci 2007; 274(1629):3079-85. doi: 10.1098/rspb.2007.0711 [Crossref] [ Google Scholar]
- Libro S, Kaluziak ST, Vollmer SV. RNA-seq profiles of immune related genes in the staghorn coral Acropora cervicornis infected with white band disease. PLoS One 2013; 8(11):e81821. doi: 10.1371/journal.pone.0081821 [Crossref] [ Google Scholar]
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell 1997; 88(3):347-54. doi: 10.1016/s0092-8674(00)81873-5 [Crossref] [ Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 1998; 281(5381):1312-6. doi: 10.1126/science.281.5381.1312 [Crossref] [ Google Scholar]
- Azmi S, Dinda AK, Chopra P, Chattopadhyay TK, Singh N. Bcl-2 expression is correlated with low apoptotic index and associated with histopathological grading in esophageal squamous cell carcinomas. Tumour Biol 2000; 21(1):3-10. doi: 10.1159/000030105 [Crossref] [ Google Scholar]
- Potten CS. The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev 1992; 11(2):179-95. doi: 10.1007/bf00048063 [Crossref] [ Google Scholar]
- Williams GT. Programmed cell death: apoptosis and oncogenesis. Cell 1991; 65(7):1097-8. doi: 10.1016/0092-8674(91)90002-g [Crossref] [ Google Scholar]
- Ramdas J, Harmon JM. Glucocorticoid-induced apoptosis and regulation of NF-kappaB activity in human leukemic T cells. Endocrinology 1998; 139(9):3813-21. doi: 10.1210/endo.139.9.6180 [Crossref] [ Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25(4):402-8. doi: 10.1006/meth.2001.1262 [Crossref] [ Google Scholar]
- Ainsworth TD, Fine M, Roff G, Hoegh-Guldberg O. Bacteria are not the primary cause of bleaching in the Mediterranean coral Oculina patagonica. ISME J 2008; 2(1):67-73. doi: 10.1038/ismej.2007.88 [Crossref] [ Google Scholar]
- Roth MS, Deheyn DD. Effects of cold stress and heat stress on coral fluorescence in reef-building corals. Sci Rep 2013; 3(1):1421. doi: 10.1038/srep01421 [Crossref] [ Google Scholar]
- Mydlarz LD, Palmer CV. The presence of multiple phenoloxidases in Caribbean reef-building corals. Comp Biochem Physiol A Mol Integr Physiol 2011; 159(4):372-8. doi: 10.1016/j.cbpa.2011.03.029 [Crossref] [ Google Scholar]
- Palmer CV, McGinty ES, Cummings DJ, Smith SM, Bartels E, Mydlarz LD. Patterns of coral ecological immunology: variation in the responses of Caribbean corals to elevated temperature and a pathogen elicitor. J Exp Biol 2011; 214(Pt 24):4240-9. doi: 10.1242/jeb.061267 [Crossref] [ Google Scholar]
- Palmer CV, Bythell JC, Willis BL. Levels of immunity parameters underpin bleaching and disease susceptibility of reef corals. Faseb j 2010; 24(6):1935-46. doi: 10.1096/fj.09-152447 [Crossref] [ Google Scholar]
- Palmer CV, Traylor-Knowles N. Towards an integrated network of coral immune mechanisms. Proc Biol Sci 2012; 279(1745):4106-14. doi: 10.1098/rspb.2012.1477 [Crossref] [ Google Scholar]
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3(11):991-8. doi: 10.1038/ni1102-991 [Crossref] [ Google Scholar]
- Bellantuono AJ, Hoegh-Guldberg O, Rodriguez-Lanetty M. Resistance to thermal stress in corals without changes in symbiont composition. Proc Biol Sci 2012; 279(1731):1100-7. doi: 10.1098/rspb.2011.1780 [Crossref] [ Google Scholar]
- Raftos DA, Kuchel R, Aladaileh S, Butt D. Infectious microbial diseases and host defense responses in Sydney rock oysters. Front Microbiol 2014; 5:135. doi: 10.3389/fmicb.2014.00135 [Crossref] [ Google Scholar]
- Moya A, Sakamaki K, Mason BM, Huisman L, Forêt S, Weiss Y. Functional conservation of the apoptotic machinery from coral to man: the diverse and complex Bcl-2 and caspase repertoires of Acropora millepora. BMC Genomics 2016; 17:62. doi: 10.1186/s12864-015-2355-x [Crossref] [ Google Scholar]
- Lasi M, Pauly B, Schmidt N, Cikala M, Stiening B, Käsbauer T. The molecular cell death machinery in the simple cnidarian Hydra includes an expanded caspase family and pro-and anti-apoptotic Bcl-2 proteins. Cell Res 2010; 20(7):812-25. doi: 10.1038/cr.2010.66 [Crossref] [ Google Scholar]
- Huettenbrenner S, Maier S, Leisser C, Polgar D, Strasser S, Grusch M. The evolution of cell death programs as prerequisites of multicellularity. Mutat Res 2003; 543(3):235-49. doi: 10.1016/s1383-5742(02)00110-2 [Crossref] [ Google Scholar]
- Roff G, Kvennefors ECE, Ulstrup KE, Fine M, Hoegh-Guldberg O. Coral disease physiology: the impact of Acroporid white syndrome on Symbiodinium. Coral Reefs 2008; 27(2):373-7. doi: 10.1007/s00338-007-0339-2 [Crossref] [ Google Scholar]
- Béraud E, Gevaert F, Rottier C, Ferrier-Pagès C. The response of the scleractinian coral Turbinaria reniformis to thermal stress depends on the nitrogen status of the coral holobiont. J Exp Biol 2013; 216(Pt 14):2665-74. doi: 10.1242/jeb.085183 [Crossref] [ Google Scholar]